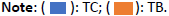Cost Effectiveness Analysis of Uterine Botulinum Toxin Injections vs. Conventional Treatment in Severe Dysmenorrhoea
Jean Martial Kouame, Eric Bautrant, Christine Leveque, Jason Robert Guertin, Melina Santos, Jessica Delorme, Oona Franke, Christophe Amiel, Thierry Bensousan, Dominique Thiers-Bautrant and Carole Siani
1Department of Pharmacy, Aix-Marseille University, Marseille, France 2Department of Pelvi-Perineal Surgery and Rehabilitation, Medical Center L'Avancée-Clinique Axium, Aix en Provence, France 3Department of Women’s Health, Women’s Health Research Center, Aix en Provence, France 4Department of Social and Preventive Medicine, Laval University, Quebec, Canada
Published Date: 2023-09-16DOI10.36648/2471-9927.9.2.76
Jean Martial Kouame1*, Éric Bautrant2,3, Christine Levêque2,3, Jason Robert Guertin4, Mélina Santos3, Jessica Delorme3, Oona Franké2,3, Christophe Amiel2,3, Thierry Bensousan2, Dominique Thiers- Bautrant2,3 and Carole Siani1
1Department of Pharmacy, Aix-Marseille University, Marseille, France
2Department of Pelvi-Perineal Surgery and Rehabilitation, Medical Center L'Avancée-Clinique Axium, Aix en Provence, France
3Department of Women’s Health, Women’s Health Research Center, Aix en Provence, Fraance
4Department of Social and Preventive Medicine, Laval University, Quebec, Canada
- *Corresponding Author:
- Jean Martial Kouame
Department of Pharmacy, Aix-Marseille University, Marseille,
France
E-mail: koffi.kouame.1@etu.univ-amu.fr
Received date: August 15, 2023, Manuscript No. IPJHME-23-17696; Editor assigned date: August 18, 2023, PreQC No. IPJHME-23-17696 (PQ); Reviewed date: September 01, 2023, QC No. IPJHME-23-17696; Revised date: September 08, 2023, Manuscript No. IPJHME-23-17696 (R); Published date: September 16, 2023, DOI: 10.36648/2471-9927.9.2.76
Citation: Kouame JM, Bautrant E, Levêque C, Guertin JR, Santos M, et al. (2023) Cost Effectiveness Analysis of Uterine Botulinum Toxin Injections vs. Conventional Treatment in Severe Dysmenorrhoea. J Health Med Econ Vol.9 No.2: 76.
Abstract
Objectives: To evaluate the efficiency of Botulinum Toxin (BT) for the management of severe dysmenorrhoea, after failure of Conventional Treatments (CT) (hormonal treatments+ analgesics) by an Incremental Cost-Effectiveness Ratio (ICER), from the perspective of the French Health Insurance (HI).
Methods: This was a retrospective study (before and after comparison) based on the patients' medical records (n=20). Data on health care consumption and quality of life were collected prospectively during the before phase, which corresponds to the CT period, and the after phase, which corresponds to the period after the addition of BT injection. The data were analyzed over a time horizon of one year, according to the perspective of the French HI. In the main analysis, total average costs included direct and indirect costs. Efficiency was assessed using the ICER. The innovative strategy was considered efficient at a threshold of 30,000 € /QALY. A probabilistic sensitivity analysis using the Monte Carlo method was performed to take into account the uncertainty around the ICER related to sampling fluctuations, as well as a deterministic sensitivity analysis to evaluate the sensitivity of the ICER to the model hypotheses.
Results: The results of the main analysis indicate, from the perspective of the French HI system, that the combination of TB+CT with an ICER: -981.98 € /QALY gained (-2187.48; 897.46), was the most efficient strategy at the 30,000 € efficiency threshold (dominant strategy). The combination of BT+CT: 714.82 € ± 336.43 € was less costly than CT alone: 1104.16 € ± 227.37 €. The main cost item in our study was the cost of daily allowances. In addition, BT was more efficient than CT in terms of QALYs gained. Therefore, the use of BT in addition to CT is an efficient and beneficial strategy that could be considered for the management of dysmenorrheic patients.
Conclusion: This study showed that, for patients with severe dysmenorrhea who are not adequately managed with CT alone, BT+CT appeared to be clinically effective and cost effective in the perspective of the French HI.
Keywords
Botulinum toxin; Cost effectiveness; Chronic pelvic pain; Dysmenorrhoea; Effectiveness; Incremental Cost-Effectiveness Ratio (ICER); Quality of life
Introduction
Dysmenorrhea is all pelvic pain preceding or accompanying menstruation. Primary dysmenorrhea affects 60%-91% of women, 16%-29% of whom have a very severe form of dysmenorrhea [1]. Severe dysmenorrhea and chronic pelvic pain of uterine origin are often accompanied by psychosocial repercussions, professional and academic absenteeism, high health care consumption and a consequent deterioration in the quality of life of the patients [2-4]. Several longitudinal studies of young dysmenorrheic women have found that absenteeism rates range from 34% to 50% and that 10% to 30% of all working and student dysmenorrheic women lose one to two working days per month [5-7]. This represents an economic burden for the community and an annual loss of 600 million work hours, or approximately $2 billion per year in the United States [8,9].
Conventional Treatment (CT) is based on analgesics and hormonal treatments, with the aim of reducing pain and promoting amenorrhea, but these treatments have proven ineffective. Faced with persistent pelvic pain, which considerably degrades the quality of life of patients. An innovative treatment, Botulinum Toxin (BT), is used in the Women’s Health Research Center (WHRC) BT in addition to CT in the management of dysmenorrhea of uterine origin related to uterine hypercontractility [10-13].
The pilot study carried out by the CRSF showed a significant reduction in pelvic pain (4.8 vs. 2.2: p=0.02) and an improvement in sexual activity [14]. In addition, 6 months after the BT injection, 14 patients (47%) had an improvement in quality of life and 12 patients (40%) received a new injection. These results were confirmed by, several studies that have shown a significant improvement in pelvic pain and quality of life after receiving uterine injections of botulinum toxin [15-23].
Beyond these promising results observed in patients with CT failure, this innovative Botulinum Toxin treatment (BT) is costly, not reimbursed by the French Health Insurance (HI), whereas it has a significant organizational and financial impact, both for the institutions and for the patients. In this context, the main objective of this cost-effectiveness study was to evaluate the efficiency of the combination of BT+CT compared to CT alone using an Incremental Cost-Effectiveness Ratio (ICER).
Methodology
Patient and method
Study population: Our study population consists of patients taken care of at the CRSF for severe dysmenorrhoeic pain, and who received an injection of Botulinum Toxin (BT) after failure of first-line CT.
The main inclusion criteria in our study were:
• Having received the injection of BT at the Women's Health Research Center (WHRC); and
• Have a medical file and questionnaires completely completed and returned. Thus, a total of 20 patients were analyzed in each group.
All women, aged ≥ 18 years old, having received uterine injection of TB for chronic pelvic pain in French gynecological center L’AVANCEE in Aix-en-Provence and having necessary data for our analysis in their medical records were enrolled.
Study design
This was a retrospective before-after study using the database of a pilot study assessing the effectiveness of uterine injection of BT in women with chronic pelvic pain (± dysmenorrhea and dyspareunia) after failure of standard treatment (hormonal and analgesic) [14].
Efficacy data: Quality-Adjusted Life Years (QALY), Patient Global Impression Improvement (PGI-I) and Visual Analogic Scale (VAS) as well as Healthcare.
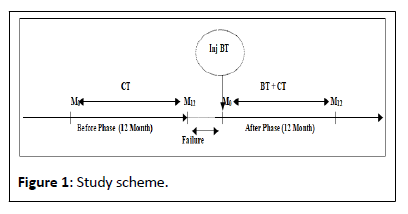
Consumption data: Medical treatments; paraclinical acts (imaging and biological examination); paramedical acts (physiotherapy and central therapy) were collected prospectively and analysed over a period of 12 months (M0 to M12) during the before phase, during which the patients received CTs, and during the after phase during which they underwent BT injection. The design of our study is schematized as follows Figure 1.
The main objective of our study was to evaluate the efficiency of BT in addition to CT compared to the conventional strategy.
Effectiveness and costs assessment
Effectiveness assessment: On the clinical level, the scale Patient Global Impression of Improvement (PGI-I) was submitted to the patients to assess their satisfaction and the scales Echelle Visuelle Analogue (EVA) in French or VAS in English VAS and EQ-5D-5L to assess their overall quality of life in relation to the treatments and their health states. The PGI-I scale is graded as follows: Much better=1; better=2; slightly better=3; no change=4; slightly worse=5; worse=6; much worse=7 and has already been used in gynecology [24]. When the patients reported a score between 1 and 2 inclusive, they were considered “satisfied” or treated successfully, otherwise “dissatisfied”. The VAS scale is scaled from 0 “worst imaginable state of health” to 100 “best imaginable state of health” [25]. The proportions of satisfied patients and the means of the VAS scores as well as the standard deviations were calculated.
The effectiveness endpoint used to assess efficiency was the QALY, measured using the EQ-5D-5L Health-related quality of life questionnaire validated within the French population [24]. He includes 5 items (mobility; autonomy; current activity; pain/ discomfort; anxiety/depression) with 5 response levels for each: No problem; mild problems; moderate problems; severe problems; extreme problems [24].
Indeed, the QALY is a composite criterion, which results from the weighting of the duration of life by the quality of life associated with this state of health. It is used in health economics to capture the effect on quality of life of various health states [26,27]. The quality of life or Utility score (U) was measured at M1 and at M12 and calculated according to the following formula: U=(1-U1-U2-U3-U4-U5). This score varies between (0 (worst state); 1 (best state)) and translates the patient's preference or not for his state of health [28].
In practice, the effectiveness QALY is obtained by multiplying the Utility score (U) calculated at M1=t1; M12=t12 by the phase duration (before and after) which is 12 months in our study, according to the following formula: QALY (t1-t12)= ((U(t1) +U(t12))/2) × (t12–t1). Where: t=time duration in a health state (in years); ex: t1–t12=12 Months=1 year; U=Utility score.
Cost assessment
The resources consumed by patients were identified and quantified (measured) retrospectively from patient files, over a time horizon of 12 months, then monetarily valued (evaluated) from the rates reimbursed by health insurance which generally correspond to 70% of the basic tariff, acts and medications.
The main costs identified in this study are as follows:
Direct costs medical: Medical consultations and paramedical acts (physiotherapy, central therapy), paraclinical acts (imaging, biological and examinations), pharmacy (drugs and medical devices), hospitalization (outpatient).
Non-medical direct costs: Transport.
Indirect costs: Daily allowances.
The perspective adopted in this study is that of French HI. In the main analysis, the total cost is equal to direct costs (medical and non-medical) added to indirect costs; thus, the resources under consideration were not restricted to the production of care (direct costs), as suggested by the High Authority of Health (HAH; or HAS in France), but extended to indirect costs, due to numerous illness-related work stoppages. However, in secondary analysis, total costs are limited to direct cost (medical and nonmedical).
Costs valorization
In practice, the costs have been valued as follows:
Direct medical costs: Hospitalization was valued from the Homogeneous Stay Group (HSG), sum paid by the HI to the establishment for the outpatient stay=300.76 Euros; the cost of acts (medical, paraclinical, paramedical) or medication equal to basic tariff of (the procedure or medication) multiplied by the reimbursed rate by HI; for example MRI=69 Euros × 70%=48.3 Euros reimbursed by HI.
Non-medical direct costs: We have valued Transport costs (TP) for hospitalization by the transport tariff reimbursed/HI. Which equal to distance travelled in kilometers multiplied by the reimbursed rate by HI, following formulae: =2* ((distance in km *0.3) *0.65).
Indirect costs: The loss of income was valued by the Daily Allowance (DA) calculated using the following formulae:
DA=Basic Daily Salary (BDS) × 50%; BDS=3 × gross salary/ 91.25. Example for a gross salary of 2000 Euros; BDS=3 × 2000/91.25=65.75; IJ=65.75 × 50%=32 Euros.
As the time horizon is not greater than 12 months, no updating has been carried out on our results, according to HAS recommendations. All costs are given in euros, relate to year 2021, and all formulae are extracted of website Ameli.fr.
Cost-effectiveness assessment
To assess the efficiency of the innovative treatment, we calculated the incremental cost-effectiveness ratio (ICER=ΔC/ΔE) of introducing BT in addition to CTs compared to CTs alone, by establishing the ratio of the mean cost difference (denoted ΔC) and the mean effectiveness difference (denoted ΔE) between both the strategies.
Otherwise, the ICER confidence interval was estimated using Fieller's theorem (Proto, 52; 50). The ceiling ratio that is the maximum ratio that community is willing to pay for a gain in effectiveness (or willingness to pay) was set at € 30,000 per QALY gained (there is no defined threshold efficiency value in the French system, for the calculation of the ICER we used values close to the threshold defined by NICE: £ 20000-£ 30000 per QALY). Then, the strategy was deemed efficient when the RCEI was less than € 30,000 per additional QALY gained [29,30].
To help institutional decision-making, we also estimated the Incremental Monetary Net Benefit (IMNB) which is equal to the difference between the mean effectiveness difference (ΔE), multiplied by the ceiling ratio (λ) and the mean cost difference (ΔC) as follows: IMNB= ΔE × λ – ΔC.
Finally, a Cost-Effectiveness Acceptability Curve (CEAC) was developed to show the probability of efficiency of the innovative strategy with respect to standard strategy according to community's willingness to pay defined by the decision maker.
Statistical analysis
Quantitative variables are described in terms of effective, mean, standard deviation and 95% confidence interval of the mean, median, range and interquartile range. Mean costs, mean QALYs and mean days off work were compared for matched subjects (before-after) by student's or Wilcoxon's test for paired series, depending on whether the distribution of variables is normal or not.
Qualitative variables are described in terms of number, percentage and 95% confidence interval according to the exact binomial distribution. The proportions of satisfied or dissatisfied Patient Global Impression of Improvement (PGI-I) were compared for the matched subjects (before-after) using Mc Nemar's chi-squared test. Descriptive analysis was performed using R studio software, Version 4.0.
The significance level was considered at 5% for the various statistical tests.
Then, sensitivity analyses were carried out on the parameters likely to impact the ICER to handle the uncertainty on the hypotheses of the model parameters. Thus, an univariate sensitivity analysis on costs and on QALYs was performed by varying different parameters. A Tornado diagram was also created to identify the most influential variables (cost) on the ICER [31,32].
Finally, the statistical uncertainty (due to sampling fluctuations) around the ICER was captured by a probabilistic sensitivity analysis, using, the Monte Carlo simulation method (article acupuncture). Therefore, the initial sample was simulated 1000 times, in order to obtain 1000 mean cost difference and mean QALY difference, the associated Incremental Cost-Effectiveness Ratios (ICERs) and the costeffectiveness acceptability curve that capture the associated uncertainty. Sensitivity analysis was performed using Excel 2017. The 95% confidence region around the ICER was also calculated using the truncated Fieller method [33].
Results
Socioeconomic characteristics
A total of 20 women were analyzed, with a mean age of 31 ± 7 years (22-46). Ten patients were in a relationship, 14 had a superior education level and 18 had a professional activity. On average, the women had 0.45 ± 0.82 children (0-3). All sociodemographic characteristics are summarized in Table 1.
| Population | n=20 |
|---|---|
| Age, mean ± SD (years) (min-max) | 31 ± 7 (22-46) |
| Marital status | |
| Single | 10 |
| Couple | 9 |
| Divorced | 1 |
| Number of children, mean ± SD (min-max) | 0.45 ± 0.82 (0-3) |
| Education level (effective) | |
| Superior (education level superior to BAC=13 years in high school) | 14 |
| Secondary (education level between 6 and 13 years) | 6 |
| Professional activity (effective) | |
| Yes | 18 |
| No (without activity) | 2 |
Table 1: Sociodemographic characteristics.
Health outcomes
The main reason for inclusion was dysmenorrheic pain. The proportion of patients who declared dysmenorrhoeic pain during the before phase was 18 (90%) against 2 (10%) during the after phase, this difference was significant (p=0.0001). We also observed a significant reduction in dyspareunia (p=0.001) and pain outside the rules (p=0.0008).
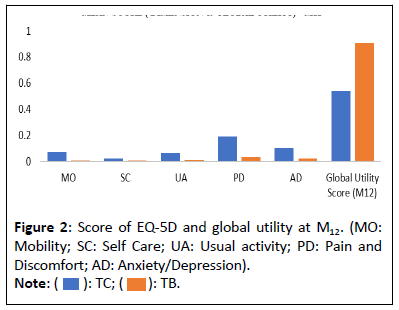
Thus, the reduction in pain results in an improvement in the quality of life of the patients and a reduction in the consumption of care a ter the BT injection. Consequently, there is a signi icant improvement in the VAS score of 49 points (p=0.00001), going from 31.6 ± 15.06 (6-70) during the before phase to 80.55 ± 18.43 (30-100) a ter the injection of BT. Moreover, the proportion of satis ied patients measured by the PGI-I scale went from 5% to 90% (p=0.0001) a ter the injection of BT. Finally, we observe an evolution of the utility score, between the phases (Before vs. A ter) respectively at M1 (0.46 vs. 0.86 (p=0.00004) and at M12 (0.54 vs. 0.91 (p=0.0002). Therefore, we can conclude from our results that BT is clinically effective compared to CT alone. Health outcomes are summarized in Table 2. Mean score of ive dimension of EQ5D and global utility score at M12 are presented in the Figure 2.
| Health outcomes | CT n=20 |
BT+CT n=20 |
p-value |
|---|---|---|---|
| Symptomatology evolution (%) | |||
| Pain during menstruation | 18 (90%) | 2 (10%) | 0.0001 |
| Dyspareunia | 15 (75%) | 3 (15%) | 0.001 |
| Pain outside menstruation | 14 (70%) | 1 (5%) | 0.0008 |
| Disabling pain/discomfort | |||
| M1 | 20 (100%) | 15 (75%) | 0.07364 |
| M12 | 20 (100%) | 12 (60%) | 0.001 |
| Score PGI-I (fréquence/M12) | |||
| Satisfied | 1(5%) | 18 (90%) | 0.0001 |
| EQ-5D VAS (means ± SD/M12) | |||
| EVA | 31.6 ± 15.06 | 80.55 ± 18.43 | 0.00001 |
| Score utility (means ± SD) | |||
| M1 | 0.46 ± 0.34 | 0.89 ± 0.18 | 0.00004 |
| M12 | 0.54 ± 0.35 | 0.91 ± 0.17 | 0.0002 |
Table 2: Health outcomes evolution.
Cost results
We observed cost differences between the main cost items, but the difference was not statistically significant for: The average cost of paramedical acts (197.38 € (119.27; 275.50) for CT vs. 123.25 € (74.40; 172.09) for BT+CT; p=0.1613); On the other hand, the differences in the average costs of para-clinical acts (€ 64.97 (47.28; 82.67) vs. € 14.20 (5.46; 22.94); p<0.0001) and the average cost of medical treatments: (€ 230.41 (17.06; 443.75) vs. 48.56 € (9.09; 88.04); p<0.0001) were statistically significant; The difference in the average costs of medical treatments is confirmed by a significant reduction in the quantities of drugs consumed (number of boxes) after injection of BT: Consumption of analgesics (25 (16.30; 33.29) vs. 6 (4.52; 8.17); p=0.0004); hormonal treatments (12 (10.26; 13.13) vs. 10 (7.68; 11.01) p=0.003) and other drugs (12 (5.77; 18.12) vs. 3 (0.88; 3.91) p=0.005).
In addition, due to disabling pain, we observed many days off work with economic losses for the community in terms of productivity losses. Thus, the loss of income of the patients is compensated by the HI via the daily allowances, which represent the indirect cost of the pathology. We note a signi icant reduction in the total number of days off work (350 days vs. 2 days, p=0.004427) and average indirect costs (€ 601.63 (-427.18; 1630.45) vs. € 4.7(-5.13; 14.53) p=0.05) a ter injection of BT.
Finally, all the costs related to dysmenorrhoeic pain, whether direct (medical and non-medical) and indirect, were included in the calculation of the total costs of the main analysis. So, we observed a difference in the average total costs, but these differences were not statistically significant between the CT group and the BT+CT injection group: € 714.82 ± € 336.43 vs. CT alone: € 1104.16 ± € 227.37 (p=0.465). Over our time horizon (12 months), the strategy (BT+CT) was less expensive than CT, this difference was mainly due to daily allowances (indirect cost). In the secondary analysis, the total cost did not include indirect costs, as suggested by the HAS, so the BT group becomes more expensive than the CT group (€ 710.12 ± € 346.23 vs. €502.32 ± € 216.23) respectively. The main cost item results are summarized in Tables 3 and 4.
| Main cost | CT n=20 means and IC 95% |
BT+CT n=20 means and IC 95% |
p-value |
|---|---|---|---|
| Cost of medical treatment | 230.41 € (17.06; 443.75) | 48.56 € (9.09; 88.04) | 0.00016 |
| Cost of clinical acts | 64.97 € (47.28; 82.67) | 14.20 € (5.46; 22.94) | 0.00065 |
| Costs of medical acts | 197.38 € (119.27; 275.50) | 123.25 € (74.40; 172.09) | 0.1613 |
| Cost of hospitalization | - | 481,21 € (373.99; 609.72) | - |
| Cost of transport | 9.15 € (-10.71; 30.31) | 32,25 € (18.51; 45.99) | 0.003 |
| Cost of daily allowance | 601,63 € (-427.18; 1630.45) | 4,72 € (-5.13; 14.53) | 0.05 |
| Total cost | 1104.16 € (786.85; 2170.47) | 714.82 € (557.37; 872.28) | 0.465 |
Table 3: Mean cost of the min expenditure items over 12 months.
| Strategy | Main analysis | Secondary analysis | ||
|---|---|---|---|---|
| Cost (€) | (QALYs) | Cost (€) | (QALYs) | |
| Innovative (BT+CT) | 714.82 € (557.37; 872.28) | 0.903 (0.831; 0.975) | 710.12 € (557.37; 872.28) | 0.903 (0.831; 0.975) |
| Standard (CT) | 1104.16 € (786.85; 2170.47) | 0.502 (0.346; 0.658) | 502.53 € (376.23; 980.84) | 0.502 (0.346; 0.658) |
| Difference | -389.34 € (-1550.97; 313.28) | 0.401 (0.250; 0.569) | 207.59 € (123.56; 465.43) | 0.401(0.265; 0.646) |
| ICER | -981.98 € /QALY (-2187,48 ; 897,46) | 517.68 € /QALY (376.23; 980.84) | ||
| IMNB | 12,430 € | 12,092.43 € | ||
Table 4: Summary of the results of the main and secondary analysis.
Effectiveness results
The endpoint used to assess effectiveness was the QALY. There was a statistically significant difference between the mean QALYs in both groups (0.502 (0.346; 0.658) for BT+CT vs. 0.903 (0.831; 0.975) for CT alone; P<0.0001). Indeed, the average QALYs after the injection of BT was higher than that of the group CT alone, showing an improvement in the quality of life of patients and the effectiveness of BT+CT.
Efficiency results: Incremental Cost Effectiveness Ratio (ICER) and Incremental Monetary Net Bene it (IMNB).
Indeed, the medico-economic results show that the management of severe dysmenorrhea by the innovative strategy including injection of BT+CT, compared to CT alone. The results of the main and secondary analysis are summarized in Table 4.
According to the cost-effectiveness plan (Figure 3) the ICER in the main analysis is located in the south east quadrant, we can then conclude that the innovative strategy is dominant (less expensive and more effective than CT alone) and should be funded by the authorities.
Sensitivity analysis
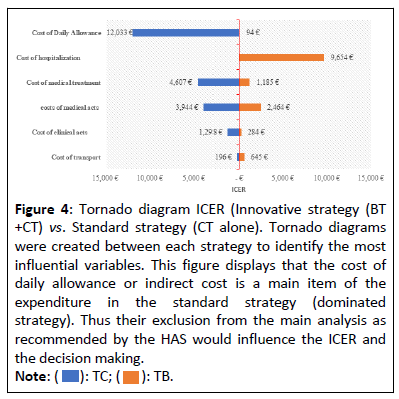
To handle the uncertainty on the hypotheses of the model parameters, the following univariate deterministic sensitivity analysis around the ICER was carried out on the parameters likely to impact this ratio in terms of decision-making and illustrated by the Tornado diagram (Figure 4), made it possible to confirm the sensitivity of the ICER to daily allowances and the need to take it into account in the main analysis.
Figure 4: Tornado diagram ICER (Innovative strategy (BT +CT) vs. Standard strategy (CT alone). Tornado diagrams were created between each strategy to identify the most influential variables. This figure displays that the cost of daily allowance or indirect cost is a main item of the expenditure in the standard strategy (dominated strategy). Thus their exclusion from the main analysis as recommended by the HAS would influence the ICER and the decision making.
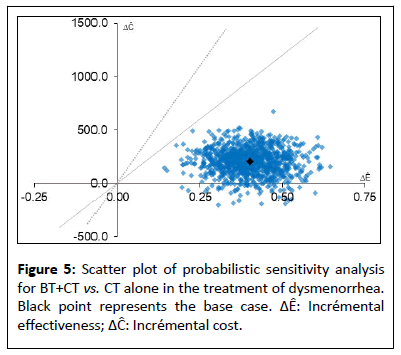
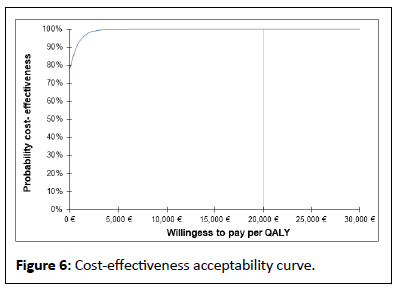
The results of the probabilistic sensitivity analysis, carried out to handle the uncertainty due to sampling fluctuations, using Monte Carlo method with 1000 simulations, are presented in a cost effectiveness plane (Figure 5). Simulations are distributed in lower and upper right quadrant. We have 95% of the simulations, in the upper right quadrant implying that BT results increase in quality of life and an increase in costs when compared with CT. In this situation, BT would be favoured according to capacity to pay of health authority. In less than 5% of proportions, the simulated ICERs appear in the lower right quadrant of the plane: The BT yields more QALYs against fewer costs than CT. In this situation, BT would clearly be favoured, being dominant (less costly end more effective). In both the scenarios the scatter plot is under the straight line of willingness to pay threshold (20,000 € and 30,000 €). Therefore, all simulations show that the innovative strategy is cost effective. The acceptability curve shows that the probability that the ICER falls below the threshold of 0 €, 20,000 € and 30,000 € in 75% and 100% respectively (Figure 6).
Cost-effectiveness acceptability curve for innovative strategy (BT+CT) in dysmenorrhea and from the perspective of HI. The results of a probabilistic analysis with 1000- time Monte Carlo simulations are illustrated by cost-effectiveness acceptability curves and demonstrated legal to 100% at a willingness to pay threshold of 20000 € -30000 € per quality-adjusted life-year gained for the innovative strategy (BT+CT).
After Probabilistic Sensitivity Analysis (PSA), the mean cost difference and the mean effects difference was respectively estimated at -400 €, 95% CI (-1550.97; 313.28) and 0.40 QALY, 95% CI (0.250; 0.569) for an ICER= -1041.52 €, 95% CI (-4185.48; 797.77) per QALY gained.
These results of the probabilistic sensitivity analysis confirm the robustness of our results. Table 5 summarizes the main results from the probabilistic sensitivity analysis.
| Strategy | Cost (€) | (QALYs) |
|---|---|---|
| Innovative (BT+CT) | 714.82 € (557.37; 872.28) | 0.903 (0.831; 0.975) |
| Standard (CT) | 1104.42 € (786.85;2170.47) | 0.502 (0.346; 0.658) |
| Difference | -400 € (-1550.97; 313.28) | 0.401 (0.250; 0.569) |
| ICER | -1041.52 € /QALY (-4185.48; 797.77) | |
| IMNB | 12,430 € | |
Table 5: Summary of results after PSA.
Discussion
The objective of this study was to evaluate the efficiency of BT addition to CT compared to CT alone by an incremental costeffectiveness ratio and to compare the evolution of medical consumption, in particular the number of work stoppages, between the phases before and after BT injection. In general, dysmenorrheic pain is accompanied by severe psycho-social repercussions and a major impact on the quality of life, productivity at work and patient care consumption [1]. The clinical results of our study showed that in patients treated with BT in addition to CT there was a significant reduction in the intensity of pain, consumption of care as well as an improvement in the quality life of patients, which reflect the effectiveness of BT compared to CT. However, we found that the effect of BT may vary from individual to individual, depending on their own clinical characteristics [34]. According to the work of Marta et al. certain comorbidities (migraines and others) were associated with a weaker response to "local" treatments [34,35]. Indeed the average duration of the effect of BT in our study was about 6 months [4-8 months]. This result is confirmed by the work of Dr. EM. Nesbitt-Hawes in which the second injection was performed at 26 weeks=6 months after the first one [17].
A priori, the price of an outpatient stay for BT injection was higher than the price of any CT; on the other hand the average total costs of the main expenditure items were lower in the BT +CT group compared to CT alone. This reduction in costs, as shown by our results, from the reduction in the consumption of treatments (analgesics and others) and above all daily allowances, the main cost item in the CT group. After one year of follow-up of the patients in the CT group, the number of lost working days was on average 17.5 days/year with an average daily allowance cost of 601 euros. Several longitudinal studies: (Andersch and Milsom, 1982; Sundel et al. 1990) carried out on young dysmenorrheic women revealed that absenteeism rates vary from 34%-50% and that 10%-30% of all dysmenorrheic women in a work situation lose one to two working days per month (12 to 24 days/year) [5,7]. This represents an annual loss of 600 million working hours, or approximately 2 billion dollars per year in the United States of America (Dawood, 1988) [9]. The results of these studies confirm the substantial economic losses linked to work stoppages that we have observed, despite the small size of our sample. However, a study on a more representative sample is necessary to estimate the annual losses in France; it will be the subject of our future research work.
To date, no study has made it possible to evaluate the efficiency and the net benefit of the association BT+CT compared to CT alone. The results of costing vary depending on the perspective and adopted analysis methodology. Indeed, when we do not include the indirect costs, in the secondary analysis in accordance with the recommendations of HAH the combination of BT+CT was found to be more expensive than CT alone (€ 710.18 vs. € 502.3, but still remained more efficient than CT alone at the € 30,000 threshold. Although, the exclusion of indirect costs does not impact the efficiency of BT in our study. Excluding indirect costs would underestimate the total cost of CT and could bias institutional decision-making since the cost of daily allowance is the main cost item in CT, as demonstrated by the univariate sensitivity analysis (Tornado diagram, Figure 3), which then justifies its inclusion in our main analysis [36]. The methodological choice to include indirect costs in our main analysis is confirmed by the work of Witt et al. 2008, who evaluate the effectiveness of acupuncture in dysmenorrheic patients [37]. As CT alone is more expensive and less effective than BT, we can conclude that injecting BT in addition to CT is the most efficient strategy. From the HI perspective, the ICER was approximately -981.98 € /QALY gained, i.e., with the association BT+CT the gain of an additional QALY would cost -981.98 € compared to the cost of the CT (i.e., a saving of 981.98 €) and the net monetary benefit would be approximately equal to 12430.41 €. The association BT+CT, is the dominate strategy.
We therefore conclusively emphasize that the introduction of BT injection into routine practice in addition to CT should be considered to reduce dysmenorrhoeic pain, to improve patient well-being and promote cost savings for the health system to date, no study confirms or invalidates the efficiency of BT, but our results are robust, as they are confirmed by the results of the probabilistic sensitivity analysis.
The main limitation of our study is related to the before and after study design (quasi-experimental method), which does not allow us to establish a causal relationship between BT and pain improvement but that was the only available data for this pathology.
In addition, the small sample size of our study (20 patients) may limit the statistical power of our results, but our results are confirmed by probabilistic sensitivity analysis and Tarazona whose study included 24 patients [34]. A randomized controlled trial is underway with a larger sample size to confirm or prove these preliminary results.
Conclusion
The introduction of BT in the management of dysmenorrhoeic is a cost effectiveness and benefic strategy. Many patients have shown significant improvements in pain symptoms, sexual function and QoL. This results in a decrease in medical consumption, which could lead to substantial savings for the health system and the community, as well as a decrease in employee absenteeism and therefore an increase in their productivity, in contrast to conventional treatments.
While awaiting the randomized controlled uteroxin study, these results are in favour of introducing BT into the treatment of severe dysmenorrhoea.
Disclosure
All authors have not none conflict of interest.
Acknowledgment
Prof. Julien Mancini, for your reviewing and inputs. Prof. Jason R Guertin, holds a research scholar award from the Fonds de recherche du Québec-Santé.
References
- Fevre A, Burette J, Bonneau S, Derniaux O, Graesslin E, et al. (2014) Dysmenorrhea. Gynecology 9: 1-10.
[Crossref], [Google Scholar]
- Ju H, Jones M, Mishra G (2014) The prevalence and risk factors of dysmenorrhea. Epidemiol Rev 36: 104-113.
[Crossref], [Google Scholar], [Indexed]
- Righarts A, Osborne L, Connor J, Gillett W (2018) The prevalence and potential determinants of dysmenorrhoea and other pelvic pain in women: A prospective study. BJOG 125: 1532-1539.
[Crossref], [Google Scholar], [Indexed]
- Tadese M, Kassa A, Muluneh AA, Altaye G (2021) Prevalence of dysmenorrhoea, associated risk factors and its relationship with academic performance among graduating female university students in Ethiopia: A cross-sectional study. BMJ Open 11: e043814.
[Crossref], [Google Scholar], [Indexed]
- Andersch B, Milsom I (1982) An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol 144: 655-660.
[Crossref], [Google Scholar], [Indexed]
- Jones AM, Nicolás AL (2004) Measurement and explanation of socioeconomic inequality in health with longitudinal data. Health Econ 13: 1015–1030.
[Crossref], [Google Scholar], [Indexed]
- Sundell G, Milsom I, Andersch B (1990) Factors influencing the prevalence and severity of dysmenorrhoea in young women. Br J Obstet Gynaecol 97: 588-594.
[Crossref], [Google Scholar], [Indexed]
- Huang G, Le AL, Goddard Y, James D, Thavorn K, et al. (2022) A systematic review of the cost of chronic pelvic pain in women. J Obstet Gynaecol Can 44: 286-293.e3.
[Crossref], [Google Scholar], [Indexed]
- Dawood MY (1988) Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. Am J Med 84: 23-29.
[Crossref], [Google Scholar], [Indexed]
- Guo SW, Mao X, Ma Q, Liu X (2013) Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of Oxytocin Receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril 991: 231-240.
[Crossref], [Google Scholar], [Indexed]
- Kataoka M, Togashi K, Kido A, Nakai A, Fujiwara T, et al. (2005) Dysmenorrhea: Evaluation with cine-mode-display MR imaging-initial experience. Radiology 235: 124-131.
[Crossref], [Google Scholar], [Indexed]
- Liu S, Zhang Q, Yin C, Liu S, Chan Q, et al. (2016) Optimized approach to cine MRI of uterine peristalsis. J Magn Reson Imaging 44: 1397-1404.
[Crossref], [Google Scholar], [Indexed]
- Lumsden MA, Baird DT (1985) Intra-uterine pressure in dysmenorrhea. Acta Obstet Gynecol Scand 64: 183-186.
[Crossref], [Google Scholar], [Indexed]
- Bautrant E, Franké O, Amiel C, Bensousan T, Thiers-Bautrant D, et al. (2021) Treatment of acute dysmenorrhoea and pelvic pain syndrome of uterine origin with myometrial botulinum toxin injections under hysteroscopy: A pilot study. J Gynecol Obstet Hum Reprod 50: 101972.
[Crossref], [Google Scholar], [Indexed]
- Spruijt MA, Klerkx WM, Kelder JC, Kluivers KB, Kerkhof MH (2022) The efficacy of botulinum toxin a injections in pelvic floor muscles in chronic pelvic pain patients: A systematic review and meta-analysis. Int Urogynecol J 33: 2951-2961.
[Crossref], [Google Scholar], [Indexed]
- Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG (2006) Botulinum toxin type a for chronic pain and pelvic floor spasm in women. Obstet Gynecol 108: 915-923.
[Crossref], [Google Scholar], [Indexed]
- Nesbitt-Hawes EM, Won H, Jarvis SK, Lyons SD, Vancaillie TG, et al. (2013) Improvement in pelvic pain with botulinum toxin type A-Single vs. repeat injections. Toxicon 63: 83-87.
[Crossref], [Google Scholar], [Indexed]
- Morrissey D, El-Khawand D, Ginzburg N, Wehbe S, O’Hare P, et al. (2015) Botulinum toxin a injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg 21: 277-282.
[Crossref], [Google Scholar], [Indexed]
- Dessie SG, von Bargen E, Hacker MR, Haviland MJ, Elkadry E (2019) A randomized, double-blind, placebo-controlled trial of onabotulinumtoxin: A trigger point injections for myofascial pelvic pain. Am J Obstet Gynecol 221: 517.e1-517.e9.
[Crossref], [Google Scholar], [Indexed]
- Adelowo A, Hacker MR, Shapiro A, Modest AM, Elkadry E (2013) Botulinum toxin type a (botox) for refractory myofascial pelvic pain. Female Pelvic Med Reconstr Surg 19: 288-292.
[Crossref], [Google Scholar], [Indexed]
- Halder GE, Scott L, Wyman A, Mora N, Miladinovic B, et al. (2017) Botox combined with myofascial release physical therapy as a treatment for myofascial pelvic pain. Investig Clin Urol 58: 134-139.
[Crossref], [Google Scholar], [Indexed]
- Jarvis SK, Abbott JA, Lenart MB, Steensma A, Vancaillie TG (2004) Pilot study of botulinum toxin type A in the treatment of chronic pelvic pain associated with spasm of the levator ani muscles. Aust N Z J Obstet Gynaecol 44: 46-50.
[Crossref], [Google Scholar], [Indexed]
- Mooney SS, Readman E, Hiscock RJ, Francis A, Fraser E, et al. (2021) Botulinum toxin A (Botox) injection into muscles of pelvic floor as a treatment for persistent pelvic pain secondary to pelvic floor muscular spasm: A pilot study. Aust N Z J Obstet Gynaecol 61: 777-784.
[Crossref], [Google Scholar], [Indexed]
- Andrade LF, Ludwig K, Goni JMR, Oppe M, Pouvourville GD (2020) A French value set for the EQ-5D-5L. PharmacoEconomics 38: 413-425.
[Crossref], [Google Scholar], [Indexed]
- Bailly E, Margulies AL, Letohic A, Fraleu-Louër B, Renouvel F, et al. (2013) Evolution of symptoms and quality of life of patients after surgery for digestive endometriosis. Gynecol Obstet Fertil 41: 627-634.
[Crossref], [Google Scholar], [Indexed]
- Bohn JA, Bullard KA, Rodriguez MI, Ecker AM (2021) Stepwise approach to the management of endometriosis-related dysmenorrhea: A cost-effectiveness analysis. Obstet Gynecol 138: 557-564.
[Crossref], [Google Scholar], [Indexed]
- (2003) World Health Organization. Making choice in health: WHO guide to cost-effectiveness analysis 2003.
- Rodrigues JC, Avila MA, Driusso P (2021) Transcutaneous electrical nerve stimulation for women with primary dysmenorrhea: Study protocol for a randomized controlled clinical trial with economic evaluation. PLoS One 16: e0250111
[Crossref], [Google Scholar], [Indexed]
- Neumann PJ, Cohen JT, Weinstein MC. (2014) Updating cost effectiveness- the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 371: 796-797.
[Crossref], [Google Scholar], [Indexed]
- Bullard KA, Edelman AB, Williams SM, Rodriguez MI (2019) Ulipristal acetate compared to levonorgestrel emergency contraception among current oral contraceptive users: A cost-effectiveness analysis. Contraception 100: 222-227.
[Crossref], [Google Scholar], [Indexed]
- Arakawa I, Momoeda M, Osuga Y, Ota I, Koga K (2018) Cost-effectiveness of the recommended medical intervention for the treatment of dysmenorrhea and endometriosis in Japan. Cost Eff Resour Alloc 16: 12.
[Crossref], [Google Scholar], [Indexed]
- Drummond MJ, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW (2015) Health gain. In: Methods for the economic evaluation of health care programmes. (4th ed), Oxford University Press, UK: 137-139.
- Siani C (2003) Which methods for calculating the confidence regions of the incremental cost-effectiveness ratio should I choose? Journal of Epidemiology and Public Health 51: 255-276.
- Tarazona-Motes M, Albaladejo-Belmonte M, Nohales-Alfonso FJ, De-Arriba M, Garcia-Casado J, et al. (2021) Treatment of dyspareunia with botulinum neurotoxin type A: Clinical improvement and influence of patients' characteristics. Int J Environ Res Public Health 18: 8783.
[Crossref], [Google Scholar], [Indexed]
- Nijs J, Leysen L, vanlauwe J, Logghe T, Ickmans K, et al. (2019) Treatment of central sensitization in patients with chronic pain: Time for change? Expert Opin Pharm 20: 1961-1970.
[Crossref], [Google Scholar], [Indexed]
- Bekkers S, Ulsen KJ, Eddy AMM, Arthur SRT, Hoogen FJA (2020) Cost-effectiveness of botulinum neurotoxin A versus surgery for drooling: A randomized clinical trial. Dev Med Child Neurol 62: 1302-1308.
[Crossref], [Google Scholar], [Indexed]
- Witt CM, Reinhold T, Brinkhaus B, Roll S, Jena S, et al. (2008) Acupuncture in patients with dysmenorrhea: A randomized study on clinical effectiveness and cost-effectiveness in usual care. Am J Obstet Gynecol 198: e1-e8.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences