Assessing the Budget Impact of Mass Spectrometry in Multiple Myeloma: A Health Economic Model from the Spanish Payer Perspective
Christian Siegfried*, Miyuru Amarapala, Noemi Puig, Sunil Lakhwani, Miguel-Teodoro Hernández and Lauren Fusfeld
Published Date: 2025-02-26Christian Siegfried1*, Miyuru Amarapala1, Noemi Puig2, Sunil Lakhwani3, Miguel-Teodoro HernaÃÂndez3, and Lauren Fusfeld1
1CRO and Consulting Services, Veranex, Boston, United States
2Department of Hematology, University Hospital of Salamanca, Salamanca, Spain
3Hospital Universitario de Canarias, University of La Laguna, Tenerife, Spain
- *Corresponding Author:
- Christian Siegfried
CRO and Consulting Services,
Veranex, Boston,
United States
E-mail: Christian.Siegfried@Veranex.com
Received date: December 26, 2024, Manuscript No. IPJHME-24-20253; Editor assigned date: December 30, 2024, PreQC No. IPJHME-24-20253 (PQ); Reviewed date: January 06, 2025, QC No. IPJHME-24-20253; Revised date: February 19, 2025, Manuscript No. IPJHME-24-20253 (R); Published date: February 26, 2025, DOI: 10.36648/2471-9927.11.1.110
Citation: Siegfried C, Amarapala M, Puig N, Lakhwani S, Hernández MT, et al. (2025) Assessing the Budget Impact of Mass Spectrometry in Multiple Myeloma: A Health Economic Model from the Spanish Payer Perspective. J Health Med Econ Vol:11 No:1
Abstract
Context: The International Myeloma Working Group (IMWG) considers mass spectrometry (MS) an appropriate substitute for serum immunofixation electrophoresis (SIFE) in multiple myeloma (MM).
Objective: We evaluated the budget impact from a Spanish payer perspective of using quantitative immunoprecipitation MS (QIP-MS) instead of SIFE to detect M-proteins in MM patients before minimal residual disease (MRD) testing.
Design, setting and participants: An excel-based model compared the testing costs of SIFE versus QIP-MS, a serumbased quantitative immunoprecipitation mass spectrometry (MS) diagnostic test, before bone marrow (BM)-based MRD testing in MM patients over two years.
Intervention: Patients eligible for autologous stem cell transplant (ASCT) had MRD testing three times in year one (following induction therapy, ASCT and consolidation) and once in year two. Patients ineligible for ASCT were tested for MRD once. Only patients with negative M-protein results received MRD tests. Parameter estimates are based on values from a targeted literature review and primary research with hematologists. A sensitivity analysis evaluated the relative importance of input parameters.
Outcome measures and results: Using QIP-MS instead of SIFE prior to MRD testing on 3,400 MM patients in Spain would reduce the number of premature MRD tests by 1,564 and save € 620,304 or € 397 per premature MRD test avoided. Net savings per QIP-MS assay would be € 89. QIPMS’s sensitivity has the greatest impact on savings.
Conclusion: This model suggests that replacing SIFE with QIP-MS prior to MRD testing could be cost saving to the payer and help patients avoid premature invasive BM aspirations.
Keywords
Multiple myeloma; Mass spectrometry; Serum immunofixation electrophoresis; Bone marrow aspiration; Minimal residual disease; Cost; Budget; Spanish National Health System (SNS)
Introduction
Multiple myeloma (MM) is a hematologic malignancy caused by the uncontrolled growth of abnormal clonal plasma cells in the bone marrow. The five-year prevalence of MM in Spain is 18.28 per 100,000 individuals [1].
To diagnose and monitor MM, clinicians identify and quantify immunoglobulins created by tumor plasma cells. These immunoglobulins are monoclonal proteins (M-proteins) which can be whole (e.g., IgG, IgA or IgM) or partial (e.g., kappa or lambda light chains) and serve as important biomarkers of disease activity. Serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE) using serum or urine (SIFE or UIFE), alongside serum measurement of free light chains (SFLC), are used to detect circulating M-proteins in patients during disease monitoring in standard clinical practice [2]. These tests, however, have limitations in detecting and quantifying low levels of M-protein during assessment of treatment response. Patients on newer more effective therapeutic regimes combining agents such as daratumumab, bortezomib, and lenalidomide may have M-protein levels that are present but undetectable by SPEP and SIFE [3]. Studies have shown that up to 31% of newly diagnosed MM (NDMM) patients who achieve CR can still be minimal residual disease (MRD) positive [4]. Additionally, these conventional tests are not always able to distinguish between endogenous M-protein and exogenous therapeutic monoclonal antibodies that are used in combination therapy for MM patients; this inability to differentiate hinders the accurate identification of complete response (CR) to therapy [5]. Compared to CR, MRD negativity is a better prognostic indicator and is associated with improved progression-free survival (PFS) and overall survival (OS) [4].
MM patients who are in CR based on IFE negativity may require bone marrow (BM) aspirations to verify the presence or absence of disease via more sensitive MRD tests, such as next-generation flow cytometry (NGF) or next-generation sequencing (NGS). NGF and NGS tests, as well as BM aspiration procedures, incur an associated cost. A blood-based disease monitoring test that is more sensitive than SIFE could potentially reduce the number of patients who undergo an invasive BM aspiration prematurely. Mass spectrometry (MS)-based serum testing is a highly sensitive technique that provides more detailed information about M-proteins in MM patients. During MM treatment response monitoring, MS has demonstrated better sensitivity in detecting M-proteins that have not been identified with conventional methods, such as SIFE [3,6]. Unlike SIFE or SPEP, the identification of specific spectra based on unique mass-to-charge (m/z) characteristics of proteins enables MS-based testing methods to distinguish therapeutic antibodies from patients’ endogenous M-proteins [6].
Acknowledging the need for MM tests with improved performance, the International Myeloma Working Group (IMWG) MS Committee reviewed the evidence on MS as a laboratory testing option [3,7]. Based on MS’ increased accuracy and improved clinical and analytical sensitivity in the detection of M-proteins, the IMWG considered MS a suitable replacement for SIFE for diagnosing and monitoring multiple myeloma and related plasma cell disorders [8]. Furthermore, the IMWG has endorsed the use of MS to aid in distinguishing therapeutic antibodies from endogenous M-proteins [3].
The immunoglobulin isotypes (GAM) for the EXENT analyser or the EXENT GAM assay (Thermo Fisher, Waltham, Massachusetts, United States), also known as quantitative immunoprecipitation mass spectrometry (QIP-MS), is a highly sensitive polyclonal antibody-based assay that is used in the detection and identification of M-proteins in the serum of patients with plasma cell disorders, including MM.
A multicenter, open-label, phase II trial of high-risk smoldering MM patients showed that QIP-MS provides increased sensitivity compared with conventional electrophoretic methods with a lower limit of measuring interval (LLMI) around ten times as sensitive as IFE at 0.015 g/L [9].
Using a simple budget impact model, this study is the first to provide a preliminary assessment of using QIP-MS instead of SIFE to identify low levels of M-proteins in MM patients and reduce the number of premature BM aspirations in Spain.
Materials and Methods
Study design
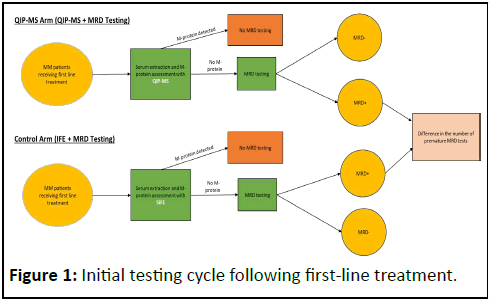
This health economic model from the Spanish payer perspective compares the budget impact of using QIP-MS instead of SIFE for M-protein testing prior to MRD testing in NDMM patients over two years. Spain has a publicly funded, highly decentralized National Health System (Sistema Nacional de Salud or SNS) that provides universal coverage through tax funding. While overseen by the Ministry of Health, administrative power is distributed across 17 autonomous communities [10]. The healthcare system operates on a global budget model with local tenders and services are funded using a global budget principle without centralized fee-for-service mechanisms or any centralized, national reimbursement/tariff coding system and schedule for diagnostics [10,11]. In this excel-based model, only patients with negative M-protein results by SIFE or QIP-MS receive MRD testing. For patients eligible for autologous stem cell transplant (ASCT), the model simulates testing they receive following induction therapy, ASCT and consolidation in the year of diagnosis, as well as testing received during the following year. The time points at which patients receive testing are based on the data from the GEM2012MENOS65 trial [12]. Patients who are ineligible for ASCT are considered for MRD testing once in the model based on clinical practice [13].
The result of the MRD test determines whether the M-protein assessment produced a true negative (i.e., the MRD assessment is negative) or a false negative (i.e., the MRD assessment is positive). Positive M-protein assessments do not result in subsequent MRD testing. The base case of the model assumes all MRD testing is performed with NGF to reflect clinical practice in Spain [13].
Figure 1 provides the framework for the initial testing cycle following first-line treatment; the patient pathway at each subsequent testing time point follows a similar pattern. The population of patients entering year two is reduced by the rate of mortality one year after initial diagnosis.
Direct medical costs in the model include the cost of M-protein tests, MRD tests, venipuncture (for M-protein testing), bone marrow aspiration (for MRD testing) and physician visits (for all testing). The model calculates total undiscounted costs related to testing.
Model development
A focused literature review was performed on PubMed for papers published since 2010 and included terms such as “multiple myeloma” and “mass spectrometry.” These papers were supplemented by an internet search for grey literature from which relevant cost and epidemiological inputs were sourced. Primary research with practicing hematologists experienced in MM management was used to develop the patient pathway and determine values for model inputs when published data were unavailable. In accordance with best practices, all clinical and cost inputs were verified by a panel of Spanish hematologists [13,14].
Clinical values
The number of MM patients in Spain receiving first-line treatment each year was estimated by multiplying the population of Spain by the incidence of MM and the proportion of patients receiving first-line therapy; the values supporting this calculation are provided in Table 1. The cohort of treated patients was then separated into two groups: Those with and without ASCT. The presence of MRD at each testing stage is the proportion of disease detected by NGF in NDMM patients in the GEM2012MENOS65 trial (69%, 50% and 44% following induction therapy, ASCT and consolidation therapy, respectively) [12]. Based on disease presence following ASCT, the model assumes 50% of ASCT patients receive consolidation therapy. The same one-year mortality rate was applied to ASCT and non-ASCT patients since the DETERMINATION trial data showed no significant difference in one-year survival in these two groups of MM patients [15].
| Parameter | Value | |
|---|---|---|
| Population of Spain | 47,222,613 [18] | |
| Incidence of Multiple Myeloma (MM) in Spain | 0.0073% [1] | |
| % of mm patients receiving 1st line therapy | 99% [13] | |
| % of Patients Receiving ASCT | 45% [13] | |
| % of MRD tests using NGF, NGS | 100%, 0% [13] | |
| Year 1 | Rate of MRD Positivity with NGF, Post-Induction | 69% [12] |
| Rate of MRD Positivity with NGF, Post-ASCT | 50% [12] | |
| Rate of MRD Positivity with NGF, Post-Consolidation | 44% [12] | |
| Year 2 | Rate of MRD Positivity with NGF | 29% [12] |
| 1 year survival rate post-diagnosis | 85% [15,19,20] | |
| Note: MM: Multiple Myeloma; ASCT: Autologous Stem Cell Transplant; MRD: Minimal Residual Disease; NGF: Next-Generation Flow Cytometry; NGS: Next-Generation Sequencing | ||
Table 1: Clinical inputs.
Sensitivity and specificity values for QIP-MS and SIFE were calculated from a study that examined testing with QIP-MS, SIFE and MRD assessment with NGS [16]. True positive and true negative results with M-protein testing from this study were determined using their concordance with results from NGS testing. These values were then used to calculate the sensitivity and specificity of QIP-MS and SIFE (Table 2). Values from the newly diagnosed secretory MM population within this study were assumed to be applicable to all MM patients within this model because about 95% to 97% of MM cases are secretory [17].
| Test specifications | QIP-MS | SIFE |
|---|---|---|
| Sensitivity | 95.5% | 54.5% |
| Specificity | 76.3% | 92.1% |
| Note: Sensitivity and specificity values for QIP-MS and SIFE were calculated using values from Derman 2021 [16]. QIP-MS: Quantitative Immunoprecipitation Mass Spectrometry, SIFE: Serum Immunofixation Electrophoresis |
||
Table 2: Sensitivity and specificity values for QIP-MS and SIFE were calculated using values from Derman 2021.
Costs
Costs are presented in Table 3. A panel of Spanish clinicians reviewed cost values and provided adjustments as needed to ensure the cost information was relevant to real-world clinical practice [13]. The cost associated with a bone marrow aspiration procedure and venipuncture costs are based on Spanish regional data. The cost of venipuncture in the literature ranges from € 6 to € 23 depending on the analysis performed [18-21]. A panel of Spanish clinicians verified the cost of venipuncture in the base case as € 17. The base case cost of testing with QIP-MS, provided by the manufacturer of the assay, is € 90 [22]. The published cost of a specialist visits in Spain in 2020 was inflated to € 94.42 in 2023 Euros using the Spanish consumer price index (CPI) of “medical and alike services” to maintain a consistent cost year across all inputs [23,24]. The same specialist visit cost was used for each of the three tests in the model: QIP-MS, SIFE and MRD testing. As recommended by Principles of Good Practice for Budget Impact Analysis II by ISPOR, total costs were not discounted [14].
| Cost category | Cost to payer |
|---|---|
| SIFE | € 10.00 |
| QIP-MS | € 90.00 |
| MRD testing with NGS | € 1,100.00 |
| MRD testing with NGF | € 300.00 |
| Venipuncture | € 17 |
| Bone marrow aspiration | € 177.00 |
| Physician visit | € 94.42 |
| Note: SIFE: Serum Immunofixation Electrophoresis; QIP-MS: Quantitative Immunoprecipitation Mass Spectrometry; MRD: Minimal Residual Disease; NGF: Next-Generation Flow Cytometry; NGS: Next-Generation Sequencing | |
Table 3: Cost inputs.
Sensitivity analyses
One-way sensitivity analyses were performed by varying each input by ± 20% of their base case value. Two scenario analyses were conducted. The first scenario examined the effect of two MRD tests for ASCT patients in the second year of the model, since clinicians indicated that the number of MRD tests in year two could range from one to two. In the second scenario, 10% of patients were allowed to receive NGS instead of NGF. The parameter for which a published range of values was available, the cost of venipuncture (€ 6 to € 23), affects both arms equally and was consequently not included in the sensitivity or scenario analyses [25].
Results
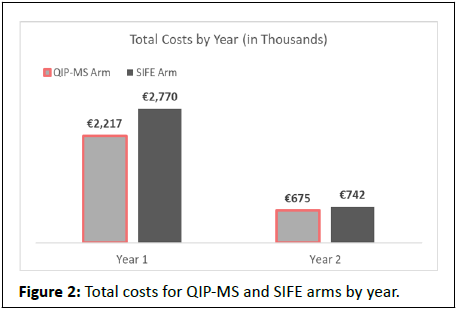
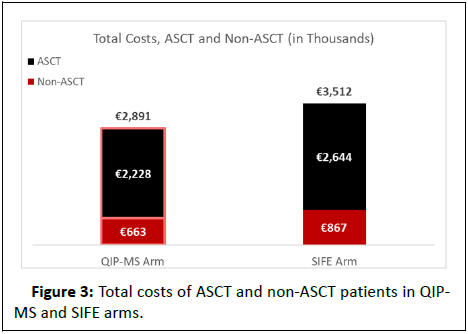
Using QIP-MS instead of SIFE prior to MRD testing on 3,400 MM patients in Spain would reduce the number of premature MRD tests by 1,564 (Table 4). Total savings would be € 620,304, with the greatest savings occurring in year one (€ 552,957) compared with year two (€ 67,346) (Table 5 and Figure 2); savings per premature MRD test avoided would be € 397. While the total cost of M-protein testing with QIP-MS is € 559,680 more than with SIFE, QIP-MS would save € 1,179,984 in MRD testing costs (inclusive of aspiration procedure and office visit costs). QIP-MS would save € 416,023 for the 1,530 ASCT patients and € 204,281 for the 1,870 non-ASCT patients in the model (Figure 3). Net savings would be € 89 per QIP-MS assay.
| Value | QIP-MS | SIFE | Difference |
|---|---|---|---|
| M-Protein tests | 6,996 | 6,996 | 0 |
| True Positives | 3,651 | 2,087 | 1,564 |
| True Negatives | 2,420 | 2,921 | -501 |
| False Positives | 751 | 250 | 501 |
| False Negatives | 174 | 1,738 | -1,564 |
| MRD tests | 2,594 | 4,659 | -2,065 |
| Note: SIFE: Serum Immunofixation Electrophoresis; QIP-MS: Quantitative Immunoprecipitation Mass Spectrometry; MRD: Minimal Residual Disease | |||
Table 4: Distribution of tests in QIP-MS and SIFE arms.
| Value | QIP-MS | SIFE | Savings |
|---|---|---|---|
| M-Protein testing | € 1,409,139 | € 849,459 | -€ 559,680 |
| MRD testing | € 1,482,265 | € 2,662,249 | € 1,179,984 |
| MRD- | € 1,382,838 | € 1,669,120 | € 286,282 |
| MRD+ | € 99,427 | € 993,129 | € 893,702 |
| Total | € 2,891,404 | € 3,511,708 | € 620,304 |
| Note: SIFE: Serum Immunofixation Electrophoresis; QIP-MS: Quantitative Immunoprecipitation Mass Spectrometry; MRD: Minimal Residual Disease | |||
Table 5: Total costs for QIP-MS and SIFE arms.
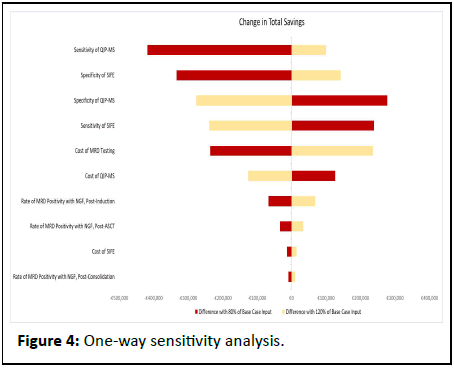
The one-way sensitivity analysis demonstrates that the most influential variables are the sensitivity and specificity of the M-protein tests (Figure 4). As an example, reducing the sensitivity of QIP-MS, the most impactful input parameter, from 95.5% to 76% corresponds to a total savings of € 202,595, which is € 417,709 lower than the savings observed in the base case of the model.
Scenario analyses
A scenario analysis was performed to measure the effect of a second testing cycle in year two for ASCT patients. In this scenario, an additional 155 premature MRD tests are avoided. Total savings are € 687,650 or € 400 per premature MRD test avoided. Additional results from this scenario analysis are presented in Table 6.
| Value | QIP-MS | SIFE | Savings |
|---|---|---|---|
| M-Protein testing | € 1,671,187 | € 1,007,427 | -€ 663,760 |
| MRD testing | € 1,894,831 | € 3,246,241 | € 1,351,410 |
| MRD- | € 1,785,690 | € 2,154,827 | € 369,138 |
| MRD+ | € 109,141 | € 1,091,413 | € 982,272 |
| Total | € 3,566,018 | € 4,253,668 | € 687,650 |
| Note: SIFE: Serum Immunofixation Electrophoresis; QIP-MS: Quantitative Immunoprecipitation Mass Spectrometry; MRD: Minimal Residual Disease | |||
Table 6: Total costs for QIP-MS and SIFE arms with two testing cycles in year two for ASCT patients.
A second scenario analysis was performed to determine the effect of using NGS and NGF to perform MRD assessments. In this scenario, 10% of MRD testing was performed using NGS.
Test results are the same as in the base case scenario, with new costs provided in Table 7.
| Value | QIP-MS | SIFE | Savings |
|---|---|---|---|
| M-Protein testing | € 1,409,139 | € 849,459 | -€ 559,680 |
| MRD testing | € 1,689,785 | € 3,034,969 | € 1,345,184 |
| MRD- | € 1,576,438 | € 1,902,800 | € 326,362 |
| MRD+ | € 113,347 | € 1,132,169 | € 1,018,822 |
| Total | € 3,098,924 | € 3,884,428 | € 785,504 |
| Note: SIFE: Serum Immunofixation Electrophoresis; QIP-MS: Quantitative Immunoprecipitation Mass Spectrometry; MRD: Minimal Residual Disease | |||
Table 7: Total costs for QIP-MS and SIFE arms with 10% and 90% of patients receiving MRD testing with NGS and NGF, respectively.
Discussion
Many MM patients routinely achieve CR as a result of recent, more effective anti-myeloma therapies. Patients in CR can be further stratified according to the depth of response using more sensitive tests, such as MRD testing [3,26]. A recent meta-analysis demonstrated that MRD negativity correlates with improved OS and PFS in multiple populations of MM patients, including high- and low-risk patients [4]. As a result of the demonstrably higher utility of these tests, MRD status (measured via NGS or NGF in the bone marrow) is increasingly used in MM clinical trials as a secondary endpoint and is expected to be used as a primary measure in the future [26]. However, MRD testing should be limited to those who do not have detectable disease in the blood; therefore, more sensitive tests that can measure M-proteins in serum are needed to guide the appropriate timing of MRD testing. These tests can also be utilized to compare the deeper responses achieved by newer, more effective, treatment regimens and distinguish these therapeutic antibodies from endogenous M-proteins [3,26].
Although international clinical guidelines indicate QIP-MS is a suitable replacement for SIFE when monitoring MM, no other studies have evaluated the budget impact of QIP-MS, which has demonstrated higher sensitivity and better agreement with MRD testing compared to standard methods [3,12,16].
This modeling exercise demonstrates that using QIP-MS instead of SIFE to inform the timing of MRD testing for MM patients could reduce the number of premature MRD procedures. The use of serum samples is more convenient and accessible for patients compared to bone marrow aspiration procedures, which are costlier, have an increased risk of complications and are often associated with pain and anxiety [8,27-29].
Limitations
While this simple model provides an initial estimate of the cost savings of QIP-MS, the study does have limitations. For example, due to a lack of longitudinal studies using MS, the model does not include data past the second year of treatment. However, the highest frequency of MRD assessments is likely to occur in the two-year timeframe of the model, with MRD testing occurring only once a year following the second year [13,30]. The model also does not consider treatment costs, treatment effects or patient outcomes, including patient quality of life, because data on treatment decisions and downstream outcomes related to QIP-MS are not yet available.
Furthermore, the model does not account for the delayed clearance of immunoglobulins. Because patients with IgG myeloma often have residual M-protein recirculating in their blood for up to six months, monitoring with single MS testing (i.e., testing only at specific milestones, such as following transplant, rather than at regular intervals) may lead to false positive test results [31,32]. This model is based on testing performed very early in the treatment lifecycle of MM patients. However, studies suggest that the number of false positives will decrease over time, with IgG MM patients and Bence Jones MM patients (who do not have this delayed clearance issue) having similar positivity rates after a year of maintenance therapy [32]. While existing evidence for QIP-MS is limited to the testing performed in trials, which often test at specific milestones, QIP-MS should be used at regular intervals (as a monitoring tool, replacing IFE) in clinical practice. As QIP-MS precisely quantifies monoclonal immunoglobulins even at low concentrations, physicians can observe downward trends in IgG levels even before QIP-MS becomes negative. In addition, published information suggests that some of the “false positive” results with MS, as determined by MRD testing, may be true positive results and these patients will progress even while patients are MRD negative with NGF. These discordant results could be due to extramedullary disease (EMD), which occurs with MM proliferation outside the BM or due to the absence of the disease at the BM extraction site, often the posterior superior iliac spine (PSIS) [33,34]. A recent abstract found MS was able to “…clearly discriminate a group of patients with inferior PFS on the group of NGF negative patients…” [34]. Mai et al. further postulate that MS positivity “…does not just reflect residual circulating monoclonal protein but derives from treatment-resistant tumor cells that constitute a source of disease relapse” [32].
Oligosecretory MM comprises approximately 2% to 3% of the MM population and the low levels of circulating M-proteins in the blood of these patients are not detected by conventional blood-based methods. Small studies in this population have demonstrated that QIP-MS is able to detect M-proteins in these patients due to its LLMI of 0.0015 g/dl, which is well below the concentration of M-proteins that defines patients as oligosecretory, 1 g/dl [35-37]. However, like other blood-based tests, QIP-MS cannot monitor disease in patients with non-secretory myeloma, which are roughly 1% to 3% of MM patients, as the disease in these patients does not secrete M-proteins into the blood.
Future studies examining real-world evidence to determine the clinical and economic impact of QIP-MS could confirm the insights demonstrated in this model and complement the limited data available on the economic burden of MM in Spain [20].
Conclusion
QIP-MS is an emerging testing modality with high clinical sensitivity in MM patients. We created a health economic tool to assess the potential budget impact of using QIP-MS to inform MRD testing in MM patients. The model, which suggests replacing SIFE with QIP-MS prior to MRD testing could be cost saving to the payer, establishes the groundwork for further research as MM treatment monitoring procedures continue to evolve.
Acknowledgments
We thank Sabrina Iadicicco (Veranex) and Sabina Schadyew (Veranex) for providing several model parameters.
Declaration of Funding
This study was funded by a research grant from Thermo Fisher Scientific.
Declaration of Financial/Other Relationships
The analysis was funded by Thermo Fisher Scientific. Veranex, Inc. received consulting fees from Thermo Fisher Scientific for developing the model on which this manuscript is based. Co-authors Christian Siegfried, Miyuru Amarapala, and Lauren Fusfeld were employed by Veranex, Inc. at the time of this research.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249
[Crossref] [Google Scholar] [PubMed]
- Multiple Myeloma Research Foundation (2024). Diagnostic testing for multiple myeloma.
- Murray DL, Puig N, Kristinsson S, Usmani SZ, Dispenzieri A, et al. (2021) Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: An International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer J 11:24
[Crossref] [Google Scholar] [PubMed]
- Munshi NC, Avet-Loiseau H, Anderson KC, Neri P, Paiva B, et al. (2020) A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv 4:5988-5999
[Crossref] [Google Scholar] [PubMed]
- Murata K, McCash SI, Carroll B, Lesokhin AM, Hassoun H, et al. (2018) Treatment of multiple myeloma with monoclonal antibodies and the dilemma of false positive M-spikes in peripheral blood. Clin Biochem 51:66-71
[Crossref] [Google Scholar] [PubMed]
- Eveillard M, Korde N, Ciardiello A, Diamond B, Lesokhin A, et al. (2021) Using MALDI-TOF mass spectrometry in peripheral blood for the follow up of newly diagnosed multiple myeloma patients treated with daratumumab-based combination therapy. Clin Chim Acta 516:136-141
[Crossref] [Google Scholar] [PubMed]
- Mills JR, Kohlhagen MC, Dasari S, Vanderboom PM, Kyle RA, et al. (2016) Comprehensive assessment of M-proteins using nanobody enrichment coupled to MALDI-TOF mass spectrometry. Clin Chem 62:1334-1344
[Crossref] [Google Scholar] [PubMed]
- Murray DL (2022) Bringing mass spectrometry into the care of patients with multiple myeloma. Int J Hematol 115:790-798
[Crossref] [Google Scholar] [PubMed]
- Berlanga O, North S, Barnidge D, Brusseau S, Patel R, et al. (2019) QIP-MS: An alternative to electrophoresis to distinguish endogenous M-proteins from therapeutic monoclonal antibodies in multiple myeloma. 19:e143-e144
- Bernal-Delgado E, Garcia-Armesto S, Oliva J, Martínez FI, Repullo JR, et al. (2018) Health system review. Health 20:1-179
[Google Scholar] [PubMed]
- Arrabal N, Escudero I, Castro A. The current situation of market access for in vitro diagnostic tests in Spain. Asebio.
- Puig N, Sanfeliciano TC, Paiva B, Cedena MT, Rosinol L, et al. (2021) Assessment of treatment response by Ife, next generation flow cytometry and mass spectrometry coupled with liquid chromatography in the GEM2012MENOS65 clinical trial. Blood 138:544
- Veranex primary research with 3 Spanish hematologists in 2024.
- Sullivan SD, Mauskopf JA, Augustovski F, Caro JJ, Lee KM, et al. (2014) Budget impact analysis-principles of good practice: Report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health 17:5-14
[Crossref] [Google Scholar] [PubMed]
- Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, et al. (2022) Triplet therapy, transplantation and maintenance until progression in myeloma. N Engl J Med 387:132-147
[Crossref] [Google Scholar] [PubMed]
- Derman BA, Stefka AT, Jiang K, McIver A, Kubicki T, et al. (2021) Measurable residual disease assessed by mass spectrometry in peripheral blood in multiple myeloma in a phase II trial of carfilzomib, lenalidomide, dexamethasone and autologous stem cell transplantation. Blood Cancer J 11:19
[Crossref] [Google Scholar] [PubMed]
- Corso A, Mangiacavalli S (2017) Non-secretory myeloma: Ready for a new definition? Mediterr J Hematol Infect Dis 9:e2017053
[Crossref] [Google Scholar] [PubMed]
- The World Factbook (2024) Washington (DC): CIA. Population of Spain.
- Survival for myeloma. London (UK): Cancer research UK. Survival statistics for myeloma.
- Ocio EM, Montes-Gaisan C, Bustamante G, Garzon S, Gonzalez E, et al. (2023) Real-world health care services utilization associated with the management of patients with relapsed and refractory multiple myeloma in spain: The CharisMMa study. Clin Lymphoma Myeloma Leuk 23:e341-e347
[Crossref] [Google Scholar] [PubMed]
- Eusko Jaurlaritza Basque Government (2023) Rates for billing of health services and Osakidetza's healthcare and educational services for the year 2024.
- Thermo Fisher Scientific Inc (2024) Assumed cost of the EXENT GAM Assay in Spain.
- Ribera Santasusana JM, de Andres Saldana A, Garcia-Munoz N, Gostkorzewicz J, Martinez Llinas D, et al. (2020) Cost-effectiveness analysis of tisagenlecleucel in the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia in children and young adults in Spain. Clinicoecon Outcomes Res 15:253-264
[Crossref] [Google Scholar] [PubMed]
- Electronic Office-National Institute of Statistics. Madrid (Spain): INE. Annual average index of medical and alike services.
- Cytometry Rates. Salamanca (Spain): Nucleus R and D.
- Yee AJ, Raje N (2021) Minimal residual disease in multiple myeloma: Why, when, where. Hematology 2021:37-45
[Crossref] [Google Scholar] [PubMed]
- Hibbs S (2022) This is going to hurt: Revisiting the patient experience of bone marrow biopsies. Hemasphere 6:e710
[Crossref] [Google Scholar] [PubMed]
- Gendron N, Zia Chahabi S, Poenou G, Rivet N, Belleville-Rolland T, et al. (2019) Pain assessment and factors influencing pain during bone marrow aspiration: A prospective study. PLoS One 14:e0221534
- Liptrott SJ, Botti S, Bonifazi F, Cioce M, de Cecco V, et al. (2021) Management of pain and anxiety during bone marrow aspiration: An Italian National Survey. Pain Manag Nurs 22:349-355
[Crossref] [Google Scholar] [PubMed]
- Ramasamy K, Avet-Loiseau H, Blimark CH, Delforge M, Gay F, et al. (2023) Measurable residual disease testing in multiple myeloma routine clinical practice: A modified delphi study. HemaSphere 7:e942
[Crossref] [Google Scholar] [PubMed]
- Abeykoon JP, Murray DL, Murray I, Jevremovic D, Otteson GE, et al. (2021) Implications of detecting serum monoclonal protein by MASS-fix following stem cell transplantation in multiple myeloma. Br J Haematol 193:380-385
[Crossref] [Google Scholar] [PubMed]
- Mai EK, Huhn S, Miah K, Poos AM, Scheid C, et al. (2023) Implications and prognostic impact of mass spectrometry in patients with newly-diagnosed multiple myeloma. Blood Cancer J 13:1
[Crossref] [Google Scholar] [PubMed]
- Blade J, Beksac M, Caers J, Jurczyszyn A, von Lilienfeld-Toal M, et al. (2022) Extramedullary disease in multiple myeloma: A systematic literature review. Blood Cancer J 12:45
- Puig N, Agullo C, Contreras T (2024) Combined mass spectrometry and next generation flow as early predictors of long-term response in relapsed/refractory myeloma patients treated with CAR-T cells and T-cell engagers.
- Giles HV, Cook MA, Drayson MT, Cook G, Wright NJ, et al. (2022) Redefining nonmeasurable multiple myeloma using mass spectrometry. Blood 139:946-950
[Crossref] [Google Scholar] [PubMed]
- Berlanga O, Simion C, Njere F, Rollins A, Panko N, et al. (2023) PB2139: Monitoring oligosecretory multiple myeloma using MALDI-TOF mass spectrometry. HemaSphere 7:e46491ff
- Migkou M, Avivi I, Gavriatopoulou M, Cohen YC, Fotiou D, et al. (2020) Clinical characteristics and outcomes of oligosecretory and non-secretory multiple myeloma. Ann Hematol 99:1251-1255
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences