An Economic Analysis of the Cost Effectiveness of Blood Gene Expression Profiling in Kidney Transplant Recipients
Roy First M1,2*, Darren Lee2, Peter Lewis2 and Stan Rose2
DOI10.21767/2471-9927.100029
Roy First M1,2*, Darren Lee2, Peter Lewis2 and Stan Rose2
1Comprehensive Transplant Center, Northwestern University, Chicago, Illinois, USA
2Transplant Genomics Inc, Mansfield, Massachusetts, USA
- *Corresponding Author:
- Roy First M
1201 W. Wrightwood Avenue
Unit 13, Chicago, Illinois, USA, 60614
Tel: (773) 677-8682
E-mail: roy@transplantgenomics.com
Received date: June 06, 2016; Accepted date: June 28, 2017; Published date: July 05, 2017
Citation: First MR, Lee D, Lewis P, Rose S (2017) An Economic Analysis of the Cost Effectiveness of Blood Gene Expression Profiling in Kidney Transplant Recipients. J Health Med Econ. Vol. 3 No. 1:3
Abstract
Background: Significant challenges exist to detecting kidney injury early in patients with kidney transplants. The current standard of care includes monitoring serum creatinine levels and immunosuppressive drug levels, both of which are poor early predictors of kidney graft damage. Protocol (surveillance) biopsies provide an accurate assessment of the transplanted kidney but are expensive, invasive, risking infection and bleeding and even graft loss, such that they are unsuited for frequent monitoring. Objectives: An economic analysis was performed to assess the economic impact of replacing protocol biopsies with blood molecular gene profiling in kidney transplant recipients. Methods: For the economic analysis, we utilized CMS fee schedule data, actual patient billing examples and published literature to estimate the per-patient tested savings of replacing protocol biopsies with the TruGraf blood test to monitor kidney transplant recipients. Results: The TruGraf test provides a net savings of $1,302 per patient per year, including the TruGraf test costs. In 2016, 19,060 kidney transplants were performed; replacing protocol biopsies with TruGraf testing could save $24.8 million in direct treatment costs per year. Conclusions: Use of the TruGraf blood test could spare patients unnecessary protocol biopsies. The healthcare system will realize significant economic benefits; in addition, the ability to intervene early with therapies to fend off clinical acute rejection may provide the added benefit of improving long term outcomes.
Keywords
Economics; Molecular gene profiling; Kidney transplantation
Introduction
In 2015, 18,587 Americans received a kidney transplant, 60% of which were Medicare patients [1]. The number of Americans living with and depending upon a functional kidney transplant is also rising. In 2015, there were over 200,000 living kidney recipients in the US, an increase of >3%/year since 2012 [2]. Results of kidney transplantation from the Scientific Registry of Transplant Recipients (SRTR) 2015 Annual Report are shown in Table 1. Short-term outcomes of kidney transplant patients have improved considerably due to an improved understanding of the immune system’s role in transplant rejection, molecular mechanisms underlying graft failure, as well as better management of immunosuppression. However, after 10 years, only 47% of deceased donor transplants and 63% of living donor transplants are still functioning [1]. As a result, 13.2% of transplants every year are re-transplants; with the unfortunate “side effect” that re-transplantation of some may deny the opportunity of ever receiving a transplant to others [1]. The US kidney transplant wait list currently contains more than 100,000 candidates, many of whom will die having never undergone a transplant [3]. In 2016, the number of kidney transplants in the US rose to 19,060 [3]. Currently, the median waiting time for a kidney is 3.6 years [4].
Table 1: Comparison of graft survival rates by donor type.
| Time after transplantation | Deceased donor | Living donor |
|---|---|---|
| 1 Year (2013) | 93.60% | 97.40% |
| 3 Years (2011) | 92.80% | |
| 5 Years (2010) | 73.60% | 85.60% |
| 10 Years (2004) | 47.20% | 62.70% |
Source: Hart et al, 2017 [1].
A key reason that graft loss remains a significant problem is because kidney injury that leads to irreversible damage, and eventual graft loss, is most often asymptomatic i.e., subclinical or chronic, for weeks and months. Patients with kidney transplants must adhere to a lifetime of immunosuppressive drug therapy to prevent their immune system from impacting graft function, which frequently manifests as acute rejection. There are significant challenges to detecting injury early when the kidney has the greatest chance of regaining normal function. The standard of care for monitoring and detecting kidney injury includes measuring serum creatinine levels, immunosuppressive drug levels and performing graft biopsies. Serum creatinine is an insensitive and lagging indicator. Drug levels may indicate potential toxicity, but are poor predictors of kidney damage. Biopsies are expensive, invasive, risking infection and bleeding and even graft loss, such that they are unsuited for frequent monitoring; moreover, significant intra-observer variation in interpretation of biopsy results exists [5]. As a consequence, using modern innovations in genomics tied to appropriate responses with immunosuppressive regimens has become a highpriority objective of transplant medicine to prevent transplant failure. Recent reviews highlighted the potential for biomarker monitoring as part of immunosuppressive therapy to improve transplant outcomes, while underscoring the need for robust multi-centre validation studies [6,7].
TruGraf™ (Transplant Genomics Inc.) is a blood test used to monitor kidney transplant recipients, providing information on adequacy of immunosuppression which may be used to support physician decisions regarding optimal therapy. TruGraf relies on gene expression signatures in blood to enable proactive non-invasive serial monitoring. The test results provide decision support for physicians in their efforts to personalize immunosuppressive therapy. The TruGraf blood test is a Laboratory Developed Test (LDT) performed as a service available exclusively through the Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory at Transplant Genomics Inc.
We have discovered and validated signatures derived from the peripheral blood of two populations of patients:
1) Patients following kidney transplantation with: (i) stable renal function, defined as a serum creatinine <2.3 mg/dL and <20% increase in serum creatinine compared to the average of 3 prior creatinine levels; and (ii) surveillance biopsies that revealed no evidence of histologic rejection. These patients were designated as TX (for “Transplant excellence”).
2) Patients following kidney transplantation not meeting the strict criteria for TX. All patients in this group underwent either surveillance or for-cause biopsies. This group included patients with stable renal function (as defined for TX) but with histological evidence of either rejection or other abnormal findings. In addition, all patients who failed to meet the definition of stable renal function were included, regardless of histological findings. These patients were designated as not-TX.
A TruGraf blood test reported as “TX” in a kidney transplant recipient would allow physicians to identify, with high probability, patients who can be followed routinely, including with serial TruGraf monitoring, without the need for an invasive surveillance biopsy. The TruGraf test is a blood-based assay that provides non-invasive, accurate assessment of adequacy of immunosuppression in kidney transplant recipients. TruGraf relies on gene-expression “signatures” that can differentiate a state of Transplant excellence (TX, indicating adequately immune-suppressed) from not-TX. As part of our CLIA laboratory test validation efforts, we evaluated the analytical performance of the blood-based TruGraf gene expression assay used to assess the adequacy of immunosuppression after kidney transplantation [8].
This manuscript describes the pharmacoeconomic analysis that we performed based on the ability of the TruGraf test to decrease the number of protocol/surveillance biopsies (the standard of care at most high volume transplant centres) in kidney transplant recipients. Our analysis utilized the Centres for Medicare and Medicaid Services (CMS) fee schedule data, actual patient billing examples and published literature to estimate the per-patient tested savings of replacing protocol biopsies with TruGraf as a kidney transplant patient monitoring test.
Methods
For the economic analysis, we utilized CMS fee schedule data, actual patient billing examples and published literature to estimate the per-patient tested savings of replacing protocol biopsies with TruGraf as a kidney transplant patient monitoring test. A conservative approach was taken and only the costs of protocol biopsies along with very minimal reductions in the incidence of acute rejection episodes, graft failures and dialysis costs due to graft failure were considered. The holistic cost of a protocol biopsy, analysis of the renal tissue sample and the associated out-patient costs was $3,931 based on the CMS Physician Fee Schedule from 2015. A typical kidney transplant protocol biopsy treatment plan consists of various combinations of protocol biopsies at 1, 3, 6 months, 1 year, and 2 years posttransplant with an average estimated usage being 1.29 per year over the first 2 years post-transplant. A subsequent biopsy was only performed when indicated by TruGraf (i.e., test results indicate not-TX and physician decides to perform biopsy). Expected incidence of not-TX detected by TruGraf was drawn from the available literature regarding rates of acute rejection of 10-15% in the first year post-transplant.
Results
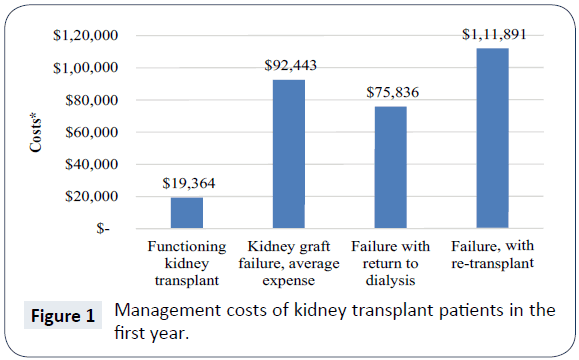
The average reimbursement for kidney transplant recipients with primary Medicare coverage totals $83,401 during the first transplant year [9]. Annual costs following the first year are smaller and are expected to remain stable throughout the patient’s life. Acute rejection is a significant contributor to individual posttransplantation costs and cost variation among transplant recipients. Data for Medicare-insured transplant recipients from 2000 to 2007 (n=45,250) drawn from the United States Renal Data System was analysed [4]. Among recipients of standard criteria donor allografts, acute rejection events were associated with significant increases in the cost of care ($17,000 to $28,000 per year). Graft failure costs an additional $66,000 [10]. When a kidney transplant fails, there are three possible outcomes: death, return to dialysis, or re-transplantation. Death is typically the least expensive outcome, because no further treatment costs are incurred. Return to dialysis and re-transplantation have staggering adverse economic consequences. In the year a kidney transplant recipient's graft fails, a 2009 study of third party payer costs revealed an average annual expense of $92,443 [11]. If the patient returns to dialysis, the average annual expense is $75,836, and, if the patient is re-transplanted, the average cost is $111,891. However, annual third party reimbursements for a patient who has a functioning kidney transplant average $19,364 [11] (Figure 1).
Our analysis utilized CMS fee schedule data, actual patient billing examples and published literature to estimate the per-patient tested savings of replacing protocol biopsies with TruGraf as a kidney transplant patient monitoring test. A conservative approach was taken and only the costs of protocol biopsies along with very minimal reductions in the incidence of acute rejection episodes, graft failures and dialysis costs due to graft failure were considered. The comprehensive cost of a single “protocol biopsy” covers a variety of services involved in obtaining the tissue sample, performing various analyses of the sample in a pathology laboratory, and related services required for patient management before, during and after the procedure. A typical bill for a protocol biopsy may include charges for an outpatient office visit, radiology/ultrasound performed in advance and during the procedure (to guide needle placement), various panels of blood tests before, during and possibly after the procedure, the surgical procedure itself (the “biopsy”), examination of the biopsy tissue in a pathology laboratory by light microscopy, fluorescence microscopy (with multiple different immunohistochemical antibodies and/or special stains employed), and/or electron microscopy, as well as all of the related professional physician fees and associated hospital fees covering space and routine services. According to the CMS Physician Fee Schedule for 2015, the comprehensive cost of a protocol biopsy, covering all of these services related to a single procedure, was $3,931 [12]. A typical kidney transplant protocol biopsy treatment plan consists of various combinations of protocol biopsies at 1, 3, 6 months, 1 year, and 2 years post-transplant with an average estimated usage being 1.29 per year over the first 2 years post-transplant. Expected incidence of not-TX detected by TruGraf was drawn from the available literature regarding rates of acute rejection and assumed to be 12% at 1 year. The baseline economic model input parameters that we employed are illustrated in Table 2.
Table 2: Baseline economic model input parameters.
| Input parameters | Baseline values |
|---|---|
| Clinical variables | |
| Number of protocol biopsies, per year | 1.29 |
| Number of acute rejection, per year | 12.00% |
| Number of graft failures, per year | 7.00% |
| Patients returning to dialysis, per year | 5.00% |
| Test utilization | |
| Number of tests per patient per year | 4 |
| Test effect | |
| Reduction in protocol biopsies | 90% |
| Reduction in acute rejection, year 1 | 5% |
| Reduction in graft failure, year 1 | 25% |
| Reduction in dialysis | 10% |
| Unit costs | |
| Cost of protocol biopsies, associated therapy | $3,931 |
| Cost of acute rejection, year 1 | $23,321 |
| Cost of graft failure, year 1 | $82,765 |
| Cost of dialysis/year | $75,000 |
Incorporating TruGraf into the current standard of care for kidney transplant patients is expected to decrease the number of biopsies and hospitalizations, and potentially improve long-term outcomes. As previously discussed, kidney transplantation and subsequent rejection and graft failure require costly therapies including biopsy, hospital admission, and increased pharmacy prescriptions. The total difference in treatment costs shows that using the TruGraf regimen may result in $6509 savings per year gross versus using protocol biopsies, or $1302 net per year including the cost of the TruGraf tests. On a per test basis this equates to savings of $325 per test or $24.8 million per year if applied across an entire 19,060 annual kidney transplant patient pool. Results of the pharmacoeconomic analysis are shown in Table 3.
Table 3: Pharmacoeconomic analysis.
| Costs | Current | With TruGraf | Difference |
|---|---|---|---|
| Protocol Bx | $5,5051 | $505 | $4,546 |
| AR | $2,799 | $2,659 | $140 |
| Graft Failure | $5,794 | $4,345 | $1,448 |
| Dialysis | $3,750 | $3,375 | $375 |
| Total (per patient tested) Gross Delta | $6,509 | ||
| Breakeven price per test | $1,627 | ||
| Total savings @ List Price (per patient tested) Net | $1,302 | ||
| Total Savings per test | $325 | ||
Discussion
The TruGraf blood test relies on the simultaneous measurement of expression data from a large number of genes, to determine whether the peripheral blood expression profile from an individual patient resembles that derived from a large reference database of kidney transplant recipients with biopsy-confirmed precise clinical phenotypes. The individual patient’s blood gene expression profile, or “signature”, is classified into one of these clinical phenotypes, providing clinicians with information to support their decisions regarding the management of immunosuppression. The TruGraf assay provides a non-invasive, accurate assessment of adequacy of immunosuppression in kidney transplant recipients. TruGraf relies on gene-expression “signatures” that can differentiate a state of Transplant excellence (TX, indicating adequately immune-suppressed) from not-TX.
TruGraf is a qualitative, “rule in/ rule out” assay. An example of a comparable commercially available assay involving a different condition is Xpresys Lung, a molecular blood test for non-invasive assessment of pulmonary nodules [13]. This test provides molecular evidence for classifying nodules as likely benign, allowing physicians to identify, with high probability, patients that are candidates for serial CT monitoring, thereby avoiding unnecessary invasive procedures. A positive test result is reported as “Likely Benign.” Physicians use this objective information in combination with standard clinical care for non-invasive assessment of lung nodules. In a similar manner, a TruGraf blood test reported as “TX” in a kidney transplant recipient would allow physicians to identify, with high probability, patients who can be followed routinely, including with serial TruGraf monitoring, without the need for an invasive surveillance biopsy. In addition, when reducing immunosuppression in the normal course of following a patient post-transplant, a signature of “TX” may reassure the clinician that there is no impending rejection as a result of reduction in immunosuppression. Conversely, a signature of “not-TX”, whether obtained in the process of monitoring a patient with stable renal function, or following reduction in immunosuppression, might support a physician decision to monitor the patient more closely, perhaps to reverse the reduction in immunosuppression, and if indicated, to perform a biopsy.
The standard of care for kidney transplant recipients includes tracking serum creatinine levels that are insensitive as well as lagging indicators of graft injury, measuring drug levels that do not reliably predict transplant outcomes, and performing invasive biopsies that are unsuited for frequent monitoring. As a result, significant tissue injury can progress for months to years without being detected or treated accordingly and result ultimately in graft failure and return to dialysis or death. Through differential diagnosis of TX versus not-TX, the TruGraf blood test provides a non-invasive means of testing when patients are not rejecting, which could be utilized for surveillance of stable patients with greater frequency and/or at different time points compared with protocol biopsies, and could have a major positive impact on patient care, enable early intervention, reduce the number of unnecessary protocol biopsies, and potentially extend graft lives and keep patients off dialysis and thereby, significantly impact healthcare savings.
The annualized cost of transplantation over 10 years is less than 25% of the cost of dialysis ($16,844 versus $70,581) [11]. Solving the problem of graft loss would create savings to payers (public and private combined) of more than $3.4 billion per year [2]. Assuming that TruGraf will lead to 90% fewer protocol biopsies per patient resulting in a saving of $4,546 per year, without incorporating the cost of TruGraf. Additional savings are obtained by factoring in the reduction in annual cost-per-patient savings due to reductions in the occurrences of acute rejection episodes, graft failures and graft failure patients who return to dialysis. Patient monitoring with TruGraf has the potential to enable physicians to not only eliminate the need for protocol biopsies, but improve patient management, reduce acute rejection episodes, graft failures due to acute rejection or nephrotoxicity and subsequent graft failures that result in patients returning to dialysis. We have conservatively estimated the following: Of the 12% of patients who experience an acute rejection episode in the first 2 years, utilization of the TruGraf test would reduce these occurrences by 5%; of the 7% of all patients who experience graft failure, it would reduce these occurrences by 25%; of the 5% of patients who experience graft loss and return to dialysis, it would reduce these occurrences by 10%. Under these assumptions, the combined savings utilizing the Trugraf test would provide a net savings of $1,302 per patient per year, including the TruGraf test costs. In 2016, 19,060 kidney transplants were performed; replacing protocol biopsy with TruGraf testing could save $24.8 million in direct treatment costs per year.
In conclusion, TruGraf blood testing provides a novel approach via non-invasive serial monitoring of kidney transplant patients to detect indicators of adequacy of immunosuppression, which previously was only possible with lagging late-stage biomarkers and invasive procedures. The primary intended use for the TruGraf test will be on patients with stable renal function after transplantation to determine their immune status, differentiating between a blood molecular profile consistent with a state of sufficient or over-immunosuppression (TX) or not (not-TX). Physicians will be able to use TruGraf results in combination with other laboratory test results and other clinical findings to help develop an individualized treatment plan based on each patient’s unique biology and immune activity levels. Through differential diagnosis of TX, TruGraf provides a non-invasive tool to support physicians in maintaining levels of effective immunosuppression and help guide personalized treatment plans. In the process, patients will be spared unnecessary protocol biopsies, the healthcare system will realize significant economic benefits, and the ability to intervene early with therapies to fend off clinical acute rejection may provide the added benefit of improving long term outcomes.
Conflicts of Interest
All authors are full-time employees of Transplant Genomics Inc., who developed the TruGraf test described in this manuscript.
References
- Matas AJ, Hart A, Smith JM, Skeans MA, Thompson B, et al. (2015) OPTN/SRTR 2015 Annual Data Report: Kidney. Am J Transplant 17: 21-116.
- Held PJ, McCormick F, Ojo A, Roberts JP (2016) A Cost Benefit Analysis of Government Compensation of Kidney Donors. Am J Transplant 16: 877-85.
- Organ Procurement and Transplantation Network.
- National Kidney Foundation, Organ donation and transplantation statistics.
- Mengel M, Sis B, Halloran PF (2007) SWOT analysis of Banff: strengths, weaknesses, opportunities and threats of the international Banff consensus process and classification system for renal allograft pathology. Am J Transplant 7: 2221-2226.
- Lo DJ, Kaplan B, Kirk AD (2014) Biomarkers for kidney transplant rejection. Nature Rev Nephrol 10: 215-225.
- Willis JC, Lord GM (2015) Immune biomarkers: the promises and pitfalls of personalized medicine. Nature Rev Immunol 15:323-329.
- Pierry D, McNulty M, Kurian SM (2017) Clinical and analytical performance validation of a molecular diagnostic signature in kidney transplant recipients. Arch Pathol Lab Med
- Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, et al. ( 2014) OPTN/SRTR 2012 Annual Data Report. Am J Transplantation 14: 11-44.
- Gheorghian A, Schnitzler MA, Axelrod DA, Kalsekar A, Lentine KL, et al. (2012) The implications of acute rejection and reduced allograft function on health care expenditures in contemporary US kidney transplantation. Transplantation 94: 241-249.
- Evans RW, Appelgate WA, Briscoe DM, Cohen DJ, Rorick CC, et al. (2010) Cost-related immunosuppressive medication non-adherence among kidney transplant recipients. Clin J Am Soc Nephrol 5: 2323-2328.
- Center for Medicare and Medicaid Services, Medicare Physician Fee Schedule Search. 2015.
- Vachani A, Pass HI, Rom WN, Midthun DE, Edel ES, et al. (2015) Validation of a multi-protein plasma classifier to identify benign lung nodules. J Thorac Oncol 10: 629-37.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences