A mini-review of detection techniques of Vibrio cholera
Daniel Hussien Reta1, 2* and Tesfaye Sisay Tessema2
Daniel Hussien Reta1,2* and Tesfaye Sisay Tessema2
1School of Veterinary Medicine, Wollo University, P.O. Box 1145, Dessie, Ethiopia
2Institute of Biotechnology, Addis Ababa University, P.O. Box 1176, Addis Ababa, Ethiopia
- *Corresponding Author:
- Daniel Hussien Reta
School of Veterinary Medicine, Wollo University, P.O. Box 1145, Dessie, Ethiopia
E-mail: gebrieldan@yahoo.com
Received Date: August 02, 2021; Accepted Date: August 17, 2021; Published Date: August 24, 2021
Citation: Reta DH, Tessema TS (2021) A Mini-Review of Detection Techniques of Vibrio cholerae. J Health Med Econ. Vol.7 No.5:58.
Abstract
Cholera is an acute enteric bacterial disease characterized by copious watery diarrhea with or without vomiting, severe dehydration and death if left untreated. The disease is caused Vibrio cholerae (V. cholerae) serogroups, O1 and O139 strains. The transmission of the infection exclusively occurs via ingestion of fecally contaminated water or food. To date, the world has hosted seven distinct cholera pandemics. In the developing world, particularly in low-income countries, cholera continues to be a significant cause of morbidity and mortality. The disease is endemic in Asia, Africa, South America and the Caribbean. Detection of the presence of V. cholerae in environmental or clinical samples early and accurately is pivotal for appropriate treatment, control and prevention of the disease. Here, we give a brief account of detection techniques of V. cholerae in a variety of specimen types. Detection methods of different types such as the conventional microbiological method, molecular techniques, enzyme-linked immunesorbent assay, dipstick tests, agglutination test, conglutination test and immunofluorescence assay are available. Commercially, several immunechromatographic format rapid diagnostic tests are available for the detection of V. cholerae in a variety of specimen types. It is more likely that the developing world will host series of cholera outbreaks in the years to come. Therefore, endemic countries should improve surveillance of V. cholerae, educate the public about the disease, provide clean drinking water and equip local health centers with diagnostic techniques of lower cost, less timeconsuming, easy to use and interpret.
Keywords
Vibrio cholerae; Microbiological techniques; Immunological techniques; Molecular techniques
Introduction
Cholera is an acute enteric bacterial disease characterized by copious watery diarrhea with or without vomiting, severe dehydration and death if left untreated. The disease is caused by infection of the intestine of the host with the gram-negative bacteria Vibrio cholerae (V. cholerae); strains belonging to O1 and O139 sero groups. The bacteria colonize the intestine, proliferate and produce potent cholera toxin (CT) to cause the infection. The transmission of the infection exclusively occurs via ingestion of fecally contaminated water or food and, rarely, by contact with infected individual. Several risk factors for the occurrence of cholera are identified, including lack of clean drinking water, poor hygiene, and high population densities [1-4].
Water bodies and human intestine are important habitats of V. cholerae; consequently, cholera outbreaks are associated with contaminated water supplies and food [5]. So far, the world has witnessed seven distinct cholera pandemics [2,6]. Cholera continues to be a significant cause of morbidity and mortality in the developing world [7]. Many parts of the developing world are endemic to cholera [8]. In endemic countries, 2.86 million cholera cases and around 95,000 deaths are estimated annually [9]. The outbreak of the disease is known to be associated with rainy seasons and flooding in endemic areas [1].
Since V. cholerae infected individuals are highly dehydrated due to watery diarrhea and vomiting, the treatment involves the administration of oral rehydration solution (ORS) and rest [3]. Despite the treatment of cholera is inexpensive and easy to administer [3], early and accurate detection of the pathogen in clinical specimen is of paramount importance [10]. Furthermore, early warning of an outbreak of cholera and effective control of the outbreak when it occurs rely heavily on accurate detection of V. cholerae in the clinical and environmental water samples [11,12].
The conventional bacteriological and biochemical methods have contributed tremendously for the detection of V. cholerae in samples [12-14]. These traditional methods of pathogen isolation and characterization are; nevertheless, cumbersome, timeconsuming, expensive and not feasible for field diagnosis [10, 15]. Molecular techniques such as polymerase chain reaction (PCR) [5,16] and real-time PCR [12,17] are also available for the detection of toxigenic and other virulence genes of the causative agent in in clinical and environmental samples. Several rapid immunoassays, including immunechromatographic assay [18, 19], dot enzyme-linked immunesorbent assay (ELISA) [20,21], sandwich ELISA [22], immunofluorescence assay [23,24] and coagglutination test [25,26] have been developed and used to detect toxigenic strains of V. cholerae in a variety of specimen types. Furthermore, commercial diagnostic tests are available for the detection of V. cholerae in clinical and environmental water samples. Most of these test kits are immunechromatographic format [27]. Here, we brief summarize detection methods of V. cholerae in clinical and environmental samples.
Cholera and Vibrio cholerae
Cholera is a water-borne acute diarrheal infection. The disease is characterized by profuse watery diarrhea with or without vomiting, severe dehydration and death if left untreated. The term “cholera” is derived from the Greek words cholē meaning bile and cholēdra meaning gutter. Both words refer to the loss of fluids from the body in the form of diarrhea. The pathogen responsible for this disease is toxigenic strains of Vibrio cholerae [1, 2].
Vibrio cholerae (V. cholerae) is facultative anaerobic, curved rods, motile, non-spore-forming and Gram-negative pathogenic bacterium. It belongs to the genus Vibrio. Naturally, the pathogen inhabits an aquatic environment. There are approximately 206 V. cholerae serogroups. The serogroups of V. cholerae differ significantly in their antigenic lipopolysaccharide (LPS) composition, with no apparent cross-reactivity between them. Out of these 206 serogroups, only O1 and O139 are the causative agents of cholera. Based on some phenotypic differences, V. cholerae O1 is subdivided into Classical and El Tor biotypes. Both El Tor and Classic biotypes are divided into 3 serotypes, namely, Ogawa, Inaba and Hikojima. Owing to their ability to produce cholera toxin (CT), V. cholerae O1 and O139 are distinctly different from other sero groups. Most of the manifestations of cholera are mainly due to CT [8,28-30].
Epidemiology of Cholera
Apparently, humans are the only natural host for the V. cholerae. Transmission of cholera infection occurs through ingestion of water or food contaminated with the feces of an infected individual [1-3]. Several risk factors for the occurrence of cholera are identified, including lack of clean drinking water, poor hygiene, and high population densities [1]. Furthermore, conflict, climate change, urbanization and population growth all play a significant role in increasing the risk of severe cholera outbreaks [4].
So far, the world has faced seven cholera pandemics. Out of the seven pandemics, the first six pandemics started in India and the seventh originated in Indonesia. The first cholera pandemic, known as “Asiatic cholera”, occurred from 1817 to 1823. The second pandemic started in 1829 and lasted until 1835. The third pandemic occurred from 1852 to 1863. The fourth cholera pandemic began in 1863 and lasted until 1879. The fifth pandemic started in 1881 and lasted until 1896. The sixth cholera pandemic occurred between 1899 and 1923. These pandemics spread to most parts of the world from their origin, India. The seven pandemic originated in Indonesia and subsequently spread to most parts of the world. The pandemic occurred from 1961 to date. The first six pandemics were caused by V. cholerae O1 Classical biotype. The ongoing seventh pandemic is caused by the El Tor biotype of V. cholerae serogroup O1 [2,6,31].
Cholera is still a major health problem in the developing world, particularly in low-income countries [31]. Many parts of the developing world, especially Africa [1,7,32], Asia [33,34] and the Caribbean [27] are endemic to cholera. In endemic countries, it is estimated that there are 2.86 million cholera cases and around 95,000 deaths annually [9]. In Ethiopia, Ali et al. [9] estimates 68, 805, 272 people are at risk of cholera, 275, 221 cholera cases and around 10, 458 deaths annually. It was estimated that there were 137, 611 - 412, 832 cholera cases and 2, 752 - 13, 761 deaths in Ethiopia in 2015 [2]. In 2017, 48, 617 cases of cholera and 880 deaths occurred in Ethiopia [4]. As of June 23, 2019, a total of 688 suspected and 23 confirmed cholera cases with associated 15 deaths have been reported in Ethiopia from 5 regions of Afar, Amhara, Oromia, Somali, Tigray regions and two administrative cities of Addis Ababa city and Dire Dawa [35]. From January to August 2020, at least 6, 789 suspected cases were also reported in Ethiopia [36]. V. cholerae serogroup O1 was confirmed as the cause of cholera outbreaks in Ethiopia [1,6,35,37].
Detection techniques of V. cholerae
Accurate and timely detection of V. cholerae in the clinical and environmental water samples is of paramount importance for patient management, early warning of cholera outbreak and effective control of the outbreak when it occurs [10-12]. Several diagnostic techniques are available for the detection of the pathogen in the samples, including the conventional bacteriological method, molecular techniques, agglutination test, coagglutination test, immunofluorescence assay, enzymelinked immunosorbent assay and immunochromatographic test.
Microbiological Techniques
The conventional microbiological technique remains the gold standard for laboratory detection of toxigenic V. cholerae. The traditional methods of pathogen isolation and characterization involve culture (stool, rectal swab and environmental samples) in artificial media, Gram staining and biochemical tests. The culture media that are used for the isolation of pathogenic V. cholerae include blood agar and MacConkey agar, the selective media being the thiosulfate-citrate-bile salts agar (TCBS) and taurocholate tellurite gelatin agar (TTGA). Alkaline peptone water (APW) is used for the transport and enrichment of V. cholerae samples. Overnight colonies of V. cholerae are small (1-3 mm), translucent, colorless-to-light pink (lactose-negative) on MacConkey agar. Overnight growth of V. cholerae on TCBS agar appears as large (2-4 mm), yellow (sucrose-positive), round, smooth, glistening, and slightly flattened. On TTGA agar, colonies of V. cholerae appear as grey, flattened, and are surrounded by a cloudy halo formed by the production of gelatinase after 24 hours of culture. The pathogen is gram negative and appears curved rod under the microscope. Several biochemical tests, including oxidase test, Kligler’s iron agar (KIA), triple sugar iron agar (TSI), Voges-Proskauer (VP) and string test can be used for the initial screening of isolates resembling V. cholerae from culture media. V. cholerae gives a positive test in the oxidase and string tests. Most isolates (75%) of V. cholerae are positive in the VP test. The reactions of V. cholerae on KIA are K/A (K, alkaline; A, acid), no gas and no H2S. On TSI, V. cholerae gives reactions of A/A (A, acid), no gas and no H2S [12-14,38-40]. The conventional microbiological methods have high specificity (100%) and provide isolates for further identification, outbreak investigations and epidemiological studies [14]. The techniques do have a number of limitations nevertheless, including being time consuming, lacking in sensitivity, laborious and require laboratory infrastructure and skilled staff [14,18,41,42].
Immunological Techniques
Agglutination Test: Since there are approximately 206 specific O antigens of V. cholerae, biochemical tests cannot be applied to differentiate V. cholerae O1/O139 from the other O serogroups. Serologic identification of V. cholerae O1/O139 serogroup is conducted by agglutination test with the specific antisera or monoclonal antibody (MAb). The test can be performed on a clean glass slide or in a petri dish. The O1 strain of V. cholerae is confirmed by agglutination with polyvalent antisera or MAb against the O1 antigen, whereas agglutination with polyvalent antisera or MAb against O139 antigen confirms the O139 strain [10,30,38,39,43]. Anti-O1 and O139 antisera are commercially available (Mast Group and Denka Seiken, Japan). V. cholerae O1 antiserum is also commercially available for the serological identification of V. cholerae O1 (Bio-Rad, France). Agglutination test demonstrated overall sensitivity and specificity of 97% and 100%, respectively, compared with enzyme linked immunosorbent assay for the identification of V. cholerae O1 and non-O1 serogroups [44]. The assay was also performed to detect V. cholerae O1 in oysters with 100% specificity [45]. The assay is rapid, technically simple and specific method to identify V. cholerae O1/O139 serogroup [46], but it requires high quality antisera or purified antibodies [43].
Coagglutination Test (COAT): Protein A coat of Staphylococcus aureus (Cowan 1 strain) is capable of binding to the fragment crystallization (Fc) portion of immunoglobulin G (IgG). This interaction between protein A and IgG is utilized for the development of COAT. In this method, V. cholerae O1/ O139 specific IgG is adsorbed onto the surface of heat-killed Staphylococcus aureus cells while retaining its binding capacity and specificity. In a positive reaction, the binding of the antibody on the surface of Staphylococcus aureus cells to the V. cholerae will cause the formation of agglutination, which can be visually read [38,41]. COAT was used for rapid detection of V. cholerae in stool samples with overall sensitivity ranging from 92% to 100% and specificity ranging from 95.65% to 100%, compared with standard culture method [25,26,47,48]. COAT is simple, rapid, inexpensive and does not require fully trained microbiology technician, but it requires V. cholerae O1/O139 specific antibodies [25,26,43,47,48].
Direct Immunofluorescence Assay (DFA): DFA was used for direct detection of V. cholerae in clinical and environmental samples [23,24]. The technique uses fluorescein isothiocyanate conjugated anti-V. cholerae O1/O139 antibody. DFA is sensitive and highly specific [23], but it requires expensive equipment, high quality immunologic reagents and trained technicians [38].
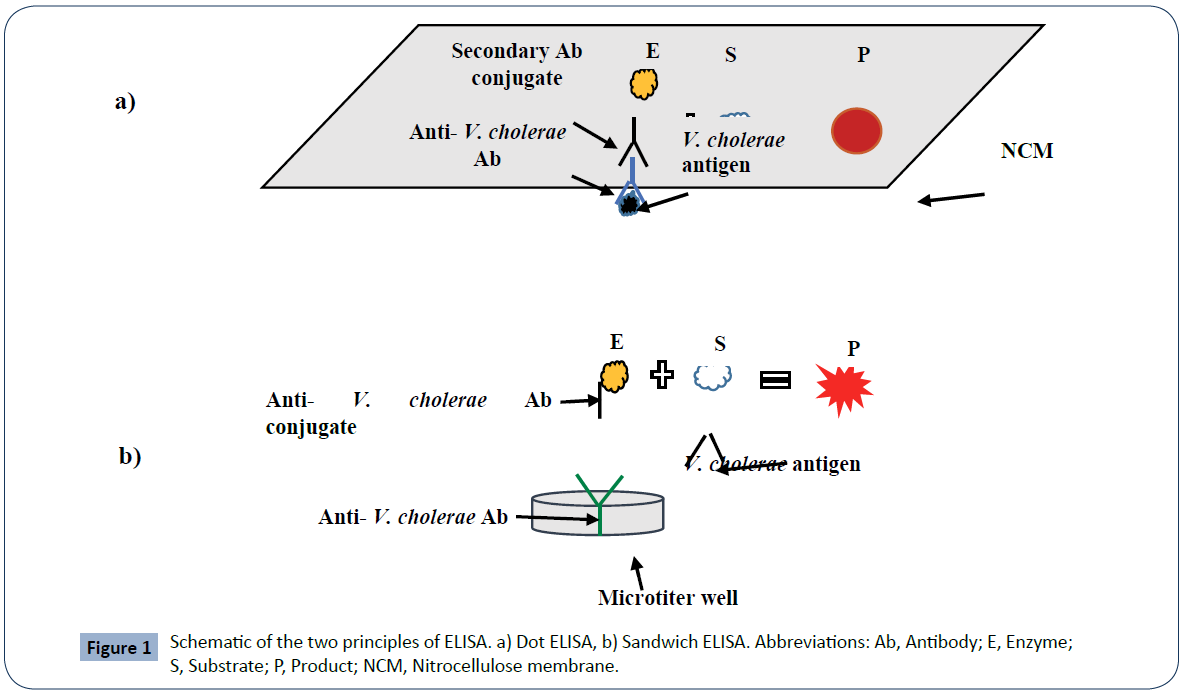
Enzyme Linked Immunosorbent Assay (ELISA): Owing to its high sensitivity and specificity, ELISA has been developed and used to detect V. cholerae or its toxin in clinical and environmental samples. Of the available variants of ELISA, dot ELISA and sandwich ELISA are commonly applied to detect V. cholerae antigens in samples. In case of dot ELISA, V. cholerae antigen is dotted onto nitrocellulose membrane. The membrane is then allowed to react with V. cholerae specific antibody and enzyme conjugated secondary antibody. The addition of a colorless chromogenic substrate leads to the formation of a colored dot on the membrane, which can be observed by the naked eye [20, 21] (Figure 1a). As illustrated in (Figure 1b), in sandwich ELISA, V. cholerae antigen first react with the immobilized antibacterial antibody and then with the second anti-bacterial antibody conjugated with enzyme. Then, addition of a colorless chromogenic substrate results in the formation of a colorful product. The color change is visually read or measured by a spectrophotometer [15]. Chaicumpa et al. [20] employed dot ELISA for the detection of V. cholerae O139 in rectal swab specimens from patients with acute watery diarrhea with overall sensitivity of 100% and specificity of 99.95%, compared with culture method. Dot ELISA was also used for the detection of V. choerae 01 in stools of diarrheic patients with 100% sensitivity and specificity, compared with culture method [49]. Sandwich ELISA was also deployed for the detection of V. cholerae and its toxin in a variety of specimen types [15,22,50]. ELISA is simple, rapid, relatively inexpensive, robust and does not require special equipment (dot ELISA) [15,20].
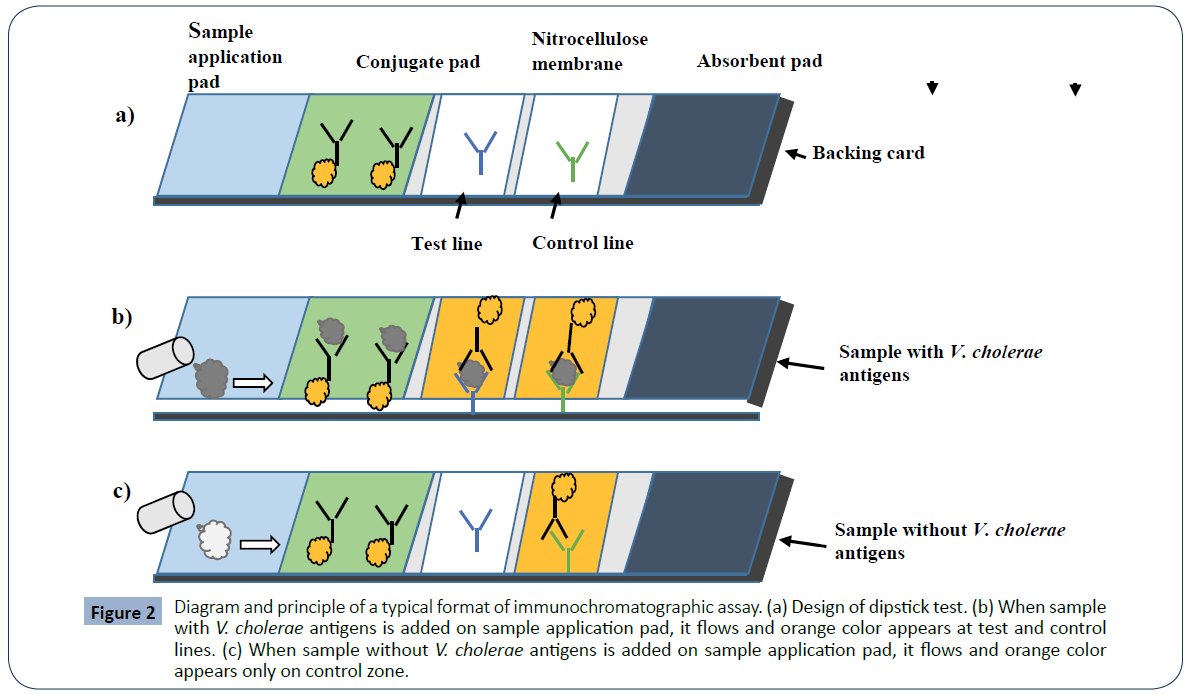
Immunochromatographic Assay (also called lateral flow assay or dipstick): A typical format of immunechromatographic assay consists of four components: sample application pad, conjugate pad, nitrocellulose membrane (test and control lines) and absorbent pad. All the four components are fixed over a backing card. The assay follows immunechromatography principle; when the sample pad of the dipstick is dipped into the sample, the aqueous sample starts to migrate towards the absorbent pad by capillary action. When the sample reaches the conjugate pad (where gold nanoparticles conjugated anti-V. cholerae O1 and/or O139 lipopolysaccharide (LPS) antibodies are immobilized), the V. cholerae antigen in the sample is captured by the immobilized antibodies and results in the formation of labeled antibody/ antigen complex. This complex then moves towards nitrocellulose membrane. At test line (where primary anti-V. cholerae O1 and/ or O139 LPS antibodies are immobilized), labeled antibody/ antigen complex is captured by the primary antibodies to produce a distinct orange line in the case of a positive reaction, or no colored line in the case of a negative result. At control line (where secondary antibody against the conjugated antibodies is immobilized), excess conjugated antibodies will be captured by the secondary antibody and gives orange line. Buffer and other excess reagents will move into the absorbent pad (Figure 2) [19, 51, 52].
Figure 2 Diagram and principle of a typical format of immunochromatographic assay. (a) Design of dipstick test. (b) When sample with V. cholerae antigens is added on sample application pad, it flows and orange color appears at test and control lines. (c) When sample without V. cholerae antigens is added on sample application pad, it flows and orange color appears only on control zone.
Immunochromatographic assay has been developed and used to detect V. cholerae O1 and O139 in clinical and environmental water samples with overall sensitivity ranging from 77.8% to 100% and specificity ranging from 84% to 100% [10, 18, 19, 53-56]. The assay is user-friendly, rapid, inexpensive and stable in different climatic conditions for prolonged periods. Consequently, the assay is potentially ideal for rapid point-of-care testing, homebased testing, and on-site testing of a variety of specimen types [19,27,51]. Several dipstick assays are commercially available, including Crystal VC Dipstick (Arkray Healthcare Pvt., India) for the detection of V. cholerae O1 and O139 in stool sample, Cholera Ag O1/O139 RDT (Standard Diagnostics Inc., Korea) for the detection of V. cholerae O1 and O139 antigens in human fecal specimens and Artron V. cholerae O139 and O1 Combo Test (Artron Laboratories Inc., Canada) for the detection of either V. cholerae O139 or O1 in human fecal samples or environmental water [27].
Molecular Techniques
Conventional polymerase chain reaction (PCR) and real-time PCR have been developed and used for characterization and confirmation of V. cholerae O1 and O139. In case of conventional PCR, the target gene is amplified exponentially using DNA polymerase and primers specific to V. cholerae. The amplified product is then visualized by gel electrophoresis method [33]. In real-time PCR, the amplification and detection activities of the target gene are conducted simultaneously. The TaqMan detector [57], molecular beacon [58] and SYBR green [59] are used to detect the amplified product. A number of toxigenic and other virulence genes of V. cholerae, including ctxA, ctxB toxR, tcpA, zot, ompU, O1-rfb, O139-rfb and ompW are targeted by these molecular techniques. For example, Yadava et al. [16] developed multiplex PCR for direct detection of V. cholerae O1 in environmental and clinical samples. This assay targeted ompW, ctxB and rfbO1 genes of V. cholerae O1. The analytical sensitivity (limit of detection; LoD) of the technique was 1.9 x 103 V. cholerae CFU per PCR reaction and no cross-reaction with other homologous bacteria used. The authors concluded that this method can be applied for V. cholerae O1 surveillance in environmental water samples and for confirmation of V. cholerae O1 in clinical samples. In one study, a multiplex PCR assay was developed for the detection of ctxA, tcpA and ompW genes of V. cholerae O1. The LoD of this assay was 8.5-85 pg bacterial genomic DNA and no cross-reaction with other related bacterial species, suggesting the utility of the technique for sensitive and specific detection of V. cholerae O1 [60]. Multiplex real-time PCR was developed for the detection of ompW, ompU, tcpA, ctxA, zot, rfbO1, and rfbO139 genes of V. cholerae with LoD of 1.4 CFU/ml in environmental water samples and no cross-reaction with other related bacterial species [17]. Gubala and Proll [58], developed multiplex real-time PCR assay for the detection of V. cholerae. The assay targeted rtxA, epsM, ompW and tcpA genes of V. cholerae. The LoD of the assay was 10 V. cholerae CFU per PCR reaction with 100% specificity. Multiplex real-time PCR was also employed for characterization of V. cholerae strains [12]. Although the molecular techniques are robust, highly specific and sensitive, they require technical expertise to run the tests, an expensive thermocycler, laboratory facilities and an expensive TaqMan detector in the case of realtime PCR [10, 15].
Conclusion
Cholera continues to be a major public health problem in many developing countries. The disease is mainly associated with poor hygiene, sanitation, limited access to clean water and high population densities. Consequently, series of cholera outbreaks will possibly be registered in most of low-income countries in the years to come. Therefore, vulnerable countries should improve surveillance of V. cholerae in a variety of specimen types, raise public awareness about the disease, improve access to clean and safe water, and equip local health centers especially peripheral laboratories with diagnostic techniques of good quality, lower cost, less time-consuming, easy to use and interpret.
References
- Bartels SA, Greenough PG, Tamar M, VanRooyen MJ (2010) Investigation of a cholera outbreak in Ethiopia's Oromiya Region. Disaster Med Public Health Prep 4: 312-317.
- Awofeso N, Aldabk K (2018) Cholera, migration, and global health – a critical review. Int J Travel Med Glob Health 6: 92-99.
- Diaconu K, Falconer J, O'May F, Jimenez M, Matragrano J, et al. (2018) Cholera diagnosis in human stool and detection in water: protocol for a systematic review of available technologies. Syst Rev 7: 29.
- Legros D (2018) Partners of the global task force on cholera control. Global cholera epidemiology: Opportunities to reduce the burden of cholera by 2030. J Infect Dis 218(suppl_3): S137-S140.
- Shanan S, Abd H, Hedenström I, Saeed A, Sandström G (2011) Detection of Vibrio cholerae and Acanthamoeba species from same natural water samples collected from different cholera endemic areas in Sudan. BMC Res Notes 4: 109.
- Scrascia M, Pugliese N, Maimone F, Mohamud KA, Ali IA, et al. (2009) Cholera in Ethiopia in the 1990 s: epidemiologic patterns, clonal analysis, and antimicrobial resistance. Int J Med Microbiol 299(5): 367-372.
- Bwire G, Sack DA, Almeida M, Li S, Voeglein JB, et al. (2018) Molecular characterization of Vibrio cholerae responsible for cholera epidemics in Uganda by PCR, MLVA and WGS. PLoS Negl Trop Dis 12: e0006492.
- (2010) The immunological basis for immunization series: module 14: cholera. World Health Organization.
- Ali M, Nelson AR, Lopez AL, Sack DA (2015) Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 9: e0003832.
- Islam MT, Khan AI, Sayeed MA, Amin J, Islam K, et al. (2019) Field evaluation of a locally produced rapid diagnostic test for early detection of cholera in Bangladesh. PLoS Negl Trop Dis 13: e0007124.
- Wang D, Xu X, Deng X, Chen C, Li B, et al. (2010) Detection of Vibrio cholerae O1 and O139 in environmental water samples by an immunofluorescent-aggregation assay. Appl Environ Microbiol 76: 5520-5525.
- Dupke S, Akinsinde KA, Grunow R, Iwalokun BA, Olukoya DK, et al. (2016) Characterization of Vibrio cholerae strains isolated from the Nigerian cholera outbreak in 2010. J Clin Microbiol 54: 2618-2621.
- Huq A, Haley BJ, Taviani E, Chen A, Hasan NA, et al. (2012) Detection, isolation, and identification of Vibrio cholerae from the environment. Curr Protoc Microbiol Chapter 6: Unit 6A.5.
- Amjad M (2020) An overview of the molecular methods in the diagnosis of gastrointestinal infectious diseases. Int J Microbiol 2020: 1-13.
- Bayat M, Khabiri A, Hemati B (2018) Development of IgY-based sandwich ELISA as a robust tool for rapid detection and discrimination of toxigenic Vibrio cholerae. Can J Infect Dis Med Microbiol 2018: 1-9.
- Yadava JP, Jain M, Goel AK (2013) Detection and confirmation of toxigenic Vibrio cholerae O1 in environmental and clinical samples by a direct cell multiplex PCR. Water SA 39: 611-614.
- Bielawska-Drózd A, Mirski T, Bartoszcze M, Cieślik P, Roszkowiak A, et al. (2012) Development of Real-Time PCR Assay for Detection of Vibrio cholerae. Pol J Environ Stud 21: 279-288.
- Debes AK, Ateudjieu J, Guenou E, Ebile W, Sonkoua IT, et al. (2016) Clinical and environmental surveillance for Vibrio cholerae in resource constrained areas: Application during a 1-year surveillance in the far north region of Cameroon. Am J Trop Med Hyg 94: 537-543.
- Sayeed MA, Islam K, Hossain M, Akter NJ, Alam MN, et al. (2018) Development of a new dipstick (Cholkit) for rapid detection of Vibrio cholerae O1 in acute watery diarrheal stools. PLoS Negl Trop Dis 12: e0006286.
- Chaicumpa W, Srimanote P, Sakolvaree Y, Kalampaheti T, Chongsa-Nguan M, et al. (1998) Rapid diagnosis of cholera caused by Vibrio cholerae O139. J Clin Microbiol 36: 3595-3600.
- Meza-Lucas A, Pérez-Villagómez MF, Martínez-López JP, García-Rodea R, Martínez-Castelán M-G, et al. (2016) Comparison of DOT-ELISA and Standard-ELISA for detection of the Vibrio cholerae toxin in culture supernatants of bacteria isolated from human and environmental samples. Indian J Microbiol 56: 379-382.
- Gustafsson B (1984) Monoclonal antibody-based enzyme-linked immunosorbent assays for identification and serotyping of Vibrio cholerae 01. J Clin Microbiol 20: 1180-1185.
- Hasan JAK, Bernstein D, Huq A, Loomis L, Tamplin ML, et al. (1994) Cholera DFA: An improved direct fluorescent monoclonal antibody staining kit for rapid detection and enumeration of Vibrio cholerae 01. FEMS Microbiol Lett 120: 143-148.
- Goel AK, Tamrakar AK, Kamboj DV, Singh L (2005) Direct immunofluorescence assay for rapid environmental detection of Vibrio cholerae O1. Folia Microbiol 50: 448-452.
- Rahman M, Sack DA, Mahmood S, Hossain A (1987) Rapid diagnosis of cholera by coagglutination test using 4-h fecal enrichment cultures. J Clin Microbiol 25: 2204-2206.
- Carillo L, Gilman RH, Mantle RE, Nunez N, Watanabe J, et al. (1994) Rapid detection of Vibrio cholerae 01 in stools of Peruvian cholera patients by using monoclonal immunodiagnostic kits. J Clin Microbiol 32: 856-857.
- Matias WR, Julceus FE, Abelard C, Mayo-Smith LM, Franke MF, et al. (2017) Laboratory evaluation of immunochromatographic rapid diagnostic tests for cholera in Haiti. PLoS ONE 12: e0186710.
- Martinez RM, Megli CJ, Taylor RK (2010) Growth and laboratory maintenance of Vibrio cholerae. Curr Protoc Microbiol.
- Seman M, Prokšová M, Rosinský J, Ferianc P (2012) Isolation, identification, and characterization of Vibrio cholerae from the Danube River in Slovakia. Folia Microbiol (Praha) 57: 191-197.
- Siriphap A, Leekitcharoenphon P, Kaas RS, Theethakaew C, Aarestrup FM, et al. (2017) Characterization and genetic variation of Vibrio cholerae isolated from clinical and environmental sources in Thailand. PLoS ONE 12: e0169324.
- Dureab F, Jahn A, Krisam J, Dureab A, Zain O, et al. (2019) Risk factors associated with the recent cholera outbreak in Yemen: a case-control study. Epidemiol Health 41: e2019015.
- Bundi M, Shah MM, Odoyo E, Kathiiko C, Wandera E, et al. (2019) Characterization of Vibrio cholerae O1 isolates responsible for cholera outbreaks in Kenya between 1975 and 2017. Microbiol Immunol 63: 350-358.
- Tamrakar AK, Jain M, Goel AK, Kamboj DV, Singh L (2009) Characterization of Vibrio cholerae from deep ground water in a cholera endemic area in Central India. Indian J Microbiol 49: 271-275.
- Rahman Z, Rahman MA, Rashid MU, Monira S, Johura FT, et al. (2018) Vibrio cholerae transmits through water among the household contacts of cholera patients in cholera endemic coastal Villages of Bangladesh, 2015-2016 (CHoBI7 Trial). Front Public Health 6: 238.
- (2019) Weekly bulletin on outbreaks and other emergencies, week 27: 1-7 July 2019. World Health Organization.
- https://reliefweb.int/sites/reliefweb.int/files/resources/humanitarian_bulletin_27_july 10_august_2020_final_for_publication.pdf
- Daniel Y (2018) Investigation of cholera outbreak kurfa chelae district, East Harer, Ethiopia, 5th September and 9th October 2017. MPH Thesis pp: 1-17.
- Kay BA, Bopp CA, Wells JG (1994) Isolation and Identification of Vibrio cholerae O1 from Fecal Specimens. Wiley Online Library.
- Kaper JB, Morris Jr JG, Levine MM (1995) Cholera. Clin Microbiol Rev 8: 48-86.
- Ramazanzadeh R, Rouhi S, Shakib P, Shahbazi B, Bidarpour F, et al. (2015) Molecular Characterization of Vibrio cholerae isolated from clinical samples in Kurdistan Province, Iran. Jundishapur J Microbiol 8: e18119.
- Ramamurthy T, Das B, Chakraborty S, Mukhopadhyay AK, Sack DA (2020) Diagnostic techniques for rapid detection of Vibrio cholerae O1/O139. Vaccine 38: A73–A82.
- Ontweka LN, Deng LO, Rauzier J, Debes AK, Tadesse F, et al. (2016) Cholera rapid test with enrichment step has diagnostic performance equivalent to culture. PLoS ONE 11: e0168257.
- https://www.cdc.gov/cholera/pdf/laboratory-methods-for-the-diagnosis-of-vibrio-cholerae-chapter 7.pdf.
- Almeida RJ, Hickman-Brenner FW, Sowers EG, Puhr ND, Farmer III JJ, et al. (1990) Comparison of a latex agglutination assay and an enzyme-linked immunosorbent assay for detecting cholera toxin. J Clin Microbiol 28: 128-130.
- Ristori CA, Rowlands REG, Jakabi M, Gelli DS, Scola MCG, et al. (2006) Use of monoclonal antibody for detecting Vibrio cholerae O1 in oysters by means of agglutination test. Rev Inst Adolfo Lutz 65: 127-132.
- Sugiyama J, Gondaira F, Matsuda J, Soga M, Terada Y (1987) New method for serological typing of Vibrio cholerae 1:0 using a monoclonal antibody-sensitized latex agglutination test. Microbiol Immunol 31: 387-391.
- Islam MM, Kabir MG, Islam AFMT, Rakib MA (2004) Coagglutination: A rapid and sensitive assay method for detection of Vibrio cholerae O1 and O139 serogroups directly from stool samples. Pak J Biol Sci 7: 1360-1364.
- Hanumanthappa AR, Rajagopal V (2001) Rapid diagnosis of cholera by coagglutination test. Indian J Pathol Microbiol 44: 123-124.
- Supawat K, Huttayananont S, Kusum M, Kalambaheti T, Chalcumpa W (1994) A monoclonal antibody-based dot-blot ELISA diagnostic kit for the detection of Vibrio cholerae O1 in stools of diarrheic patients and household contacts. Asian Pac J Allergy Immunol 12: 155-159.
- Bhadra RK, Biswas T, Pal SC, Takeda T, Nair GB (1991) A polyclonal-monoclonal antibody based sensitive sandwich enzyme linked immunosorbent assay for specific detection of cholera toxin. Zbl Bakt 275: 467-473.
- Sajid M, Kawde A, Daud M (2015) Designs, formats and applications of lateral flow assay: A literature review. J Saudi Chem Soc 19: 689-705.
- Thattiyaphong A, Okada K, Khangrang S, Nispa W, Sawanpanyalert P, et al. (2013) Development of a 5-minute rapid test for detecting Vibrio cholerae O139. Southeast Asian J Trop Med Public Health 44: 448-455.
- Chibwe I, Kasambara W, Kagoli M, Milala H, Gondwe C (2020) Field evaluation of Cholkit rapid diagnostic test for Vibrio Cholerae O1 during a cholera outbreak in Malawi, 2018. Open Forum Infect Dis 7: ofaa493.
- Chakraborty S, Alam M, Scobie HM, Sack DA (2013) Adaptation of a simple dipstick test for detection of Vibrio cholerae O1 and O139 in environmental water. Front Microbiol 4: 320.
- Bhuiyan NA, Qadri F, Faruque AS, Malek MA, Salam MA, et al. (2003) Use of dipsticks for rapid diagnosis of cholera caused by Vibrio cholerae O1 and O139 from rectal swabs. J Clin Microbiol 4: 3939-3941.
- Nato F, Boutonnier A, Rajerison M, Grosjean P, Dartevelle S, et al. (2003) One-step immunochromatographic dipstick tests for rapid detection of Vibrio cholerae O1 and O139 in stool samples. Clin Diagn Lab Immunol 10: 476-478.
- Tebbs RS, Brzoska PM, Furtado MR, Petrauskene OV (2011) Design and validation of a novel multiplex real-time PCR assay for Vibrio pathogen detection. J Food Prot 74: 939-948.
- Gubala AJ, Proll DF (2006) Molecular-beacon multiplex real-time PCR assay for detection of Vibrio cholerae. Appl Environ Microbiol 72: 6424-6428.
- Rashid RB, Ferdous J, Tulsiani S, Jensen PKM, Begum A (2017) Development and validation of a novel real-time assay for the detection and quantification of Vibrio cholerae. Front Public Health 5: 109.
- Mehrabadi JF, Morsali P, Nejad HR, Fooladi AAI (2012) Detection of toxigenic Vibrio cholerae with new multiplex PCR. J Infect Public Health 5(3): 263-267.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences