Cost-Effectiveness Analysis of Regorafenib for Third-line Metastatic Colorectal Cancer Compared to Cetuximab Plus Irinotecan in China
Shuiqing Zhu, Jin Liu, Wentao Sun, Libo Tao,Dunming Xiao and Jianwei Xuan
DOI10.21767/2471-9927.100038
1Shanghai Centennial Scientific Co. Ltd, Shanghai, China
2Bayer Healthcare Co. Ltd, China
3Zhongshan University, Guangzhou, China
- *Corresponding Author:
- Shuiqing Zhu
Shanghai Centennial Scientific Co. Ltd
Shanghai, China
Tel: 15201924528
E-mail: jwx02467@gmail.com
Received Date: May 13, 2018; Accepted Date: June 03, 2018; Published Date: July 13, 2018
Citation: Zhu S, Liu J, Sun W, Tao L, Xiao D (2018) Cost-Effectiveness Analysis of Regorafenib for Third-line Metastatic Colorectal Cancer Compared to Cetuximab Plus Irinotecan in China. J Health Med Econ Vol.4 No.1:5
DOI: 10.21767/2471-9927.100038
Abstract
Objective: While the clinical effect of regorafenib for third-line metastatic colorectal cancer has been established, the economic effect of adopting the new therapy (regorafenib) is still unclear. The present study aimed to examine the costeffectiveness of regorafenib compared to cetuximab plus irinotecan in the Chinese setting. Methods: A Markov model was constructed to conduct the cost-effectiveness analysis from a third-party payer perspective. The cost of oncology drug, utilization of both in-hospital and outpatient care facilities, administration of medications via parenteral routes, use of supportive care medications, clinical monitoring with lab tests and diagnostic imaging, and care for treatment-emergent severe adverse events were considered. Clinical effectiveness data were obtained from the clinical trials. One-way sensitivity and probability sensitivity analyses were conducted to examine the robustness of the base-case findings. Results: The model projected patients on regorafenib had an incremental gain of 0.03 QALYs relative to cetuximab plus irinotecan (0.68 Vs 0.65) at a cost-saving of ¥195,756 (¥221,860Vs¥417,616). For the subpopulation who received no previous targeted treatment, compared to cetuximab plus irinotecan, regorafenib was expected to result in additional gains of 0.15 QALYs at cost-saving of ¥95,987. Probability sensitivity analyses show that at the threshold of 3 times of GDP per capita of China (¥53,980*3), regorafenib has the probability of 82% to be costeffective against with cetuximab plus irinotecan. Conclusions: Regorafenib monotherapy is cost-saving and more effective compared with cetuximab plus irinotecan regimen in treatment patients with mCRC at third-line treatment setting in China.
Keywords
Regorafenib; Cetuximab plus irinotecan; Third-line, Metastatic colorectal cancer; Cost-effectiveness analysis
Introduction and Background
Colorectal cancer (CRC) is the third most common cancer in men and the second in women worldwide. Each year, about 1.36 million new cases have been diagnosed and nearly 700,000 patients died from colorectal cancer (CRC). In China, about 376,000 new cases per year have been diagnosed and nearly 191,000 patients died [1,2]. Approximately 25% of newly diagnosed patients have already developed metastases, and 50% of all CRC patients will develop metastases over time as the disease progresses [3].
There have been significant advances in treatment for metastatic colorectal cancer (mCRC) in the past decade. Previously, the therapy was restricted to fluoropyrimidine (5-FU)-based regimens alone or in combination with oxaliplatin or irinotecan for many years [4]. Recent development and introduction of monoclonal antibodies targeting the vascular endothelial growth factor (VEGF) and the epidermal growth factor receptor (EGFR) have brought new armamentarium to mCRC treatment and significantly improved patient survival [5-7]. However, virtually all patients would eventually become treatment refractory and experience disease progression and there remain critical unmet needs for new treatment options for patients who failed these regimens.
Regorafenib is an oral multi-kinase inhibitor that targets angiogenic, stromal, and oncogenic receptor tyrosine kinases [8] and was approved in 2017 by CFDA as a third-line therapy for the treatment of patients with metastatic colorectal cancer (CRC) who have been previously treated with, fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, and who have been previously treated with, or are not considered candidates for, an anti-VEGF therapy or an anti-EGFR therapy (if RAS wild type). The approval was based on the results of a multicountry randomized controlled phase 3 CONCUR trial, which showed statistically significant improvement with Regorafenib over placebo in overall survival (OS) in mCRC patients. In Asian patients who had received at least two previous treatment lines for mCRC or were unable to tolerate standard treatments, singleagent regorafenib improved median OS from 6.3 months in the placebo arm to 8.8 months, and 45% reduction in death (hazard ratio=0.55). In subgroup patients without prior targeted therapy, median OS was 9.7 months vs. 4.9 months (hazard ratio=0.31). The survival benefits of regorafenib in CONCUR trial was in line with previous findings of international, multi-country randomized controlled phase 3 CORRECT trial.
While regorafenib has demonstrated compelling survival advantage as a third line treatment option for mCRC patients, the greater clinical benefits must be balanced against ever increasing health care expenses. Cost-containment measures implemented by third-party payers necessary to fund public sector coverage often result in a challenging market access environment for novel medications. Inherent to this process is the consideration of costeffectiveness comparing this new regimen with other available treatment options for similarly indicated patient population. According to Chinese Standard for the Diagnosis and Treatment of Colorectal Cancer (2017), Stivarga is recommended as a standard choice for third-line treatment of mCRC, and Cetuximab plus Irinotecan may be the alternative option to be considered if the patients didn’t receive any target therapy in the first and second lines. Our objective was to examine the cost effectiveness of regorafenib as a standard third-line agent in the treatment of patients with mCRC compared with cetuximab plus irinotecan.
Materials and Methods
The study developed a Markov model of patients with mCRC in the third-line treatment setting comparing regorafenib single agent with cetuximab plus irinotecan regimen. Patients who initially received regorafenib or cetuximab plus irinotecan would terminate therapy because of disease progression or intolerance of Grade 3 to 4 adverse events (AEs). The study assumed that patients who failed third-line treatment would stop further direct anti-cancer treatment and only receive best supportive care. All patients in each health state could experience progression to death (Figure 1). The study used the exponential distribution to model survival and the model was constructed using Microsoft Excel. The primary health outcomes for the economic evaluation were life-years (LYs) and quality adjusted life-years (QALYs) gained. Cost effectiveness was calculated from third-party payer perspective in China. The simulation for each cycle is 7-day duration with half-cycle correction for health outcomes and all patients were followed from start of third-line therapy until death or end of 10th year.
Effectiveness Estimates
Effectiveness estimates for survival of each treatment group was extracted from their respective clinical trial evidence given the same baseline of population, with large representative sample and previously received the same chemotherapy regimen. The survival benefit for regorafenib monotherapy was modelled based on CONCUR trial, which was a randomized double-blind, placebo-controlled, phase 3 trial conducted in Asian countries in patients with refractory mCRC and had previously received at least 2 systemic anticancer treatment lines [9]. The median progression-free survival (PFS) and OS were 3.2 and 8.8 months, respectively.
The survival data for the combination therapy cetuximab plus irinotecan was obtained from a single arm trial of patients with mCRC previously exposed to fluoropyrimidine, irinotecan and oxaliplatin containing regimes. The median PFS and OS for patients with mutation biomarkers were 4.2 and 8.6 months, respectively.
The study also conducted cost-effectiveness evaluation in the subgroup of patients who had previously received chemotherapy only in this setting. The survival information for regorafenib and cetuximab plus irinotecan was extracted from CONCUR trials [7]. With median PFS 5.4 months and OS 9.7 months, and cetuximab plus irinotecan from BOND trials [9]. In Concur trial, with median PFS 4.1 months and OS 8.6 months, respectively.
Adverse Events
Adverse events (AEs) included in the model were based on information reported in the respective trials [9,11]. In all instances, the study limited our attention to the top five AEs of Grade 3/4 severity for each arm. Patients were assumed to be at risk for AEs only during time spent progression-free and on therapy.
Health Utilities
The estimates of health utility values at pre-progression (Stable) and post disease progression states for regorafenib were based on information reported from CONCUR trial, in which the EQ- 5D questionnaire was used to measure health utility at both baseline and at the end of treatment upon disease progression. Mean EQ-5D index scores were 0.84 at baseline and 0.57 at end of treatment. Since the study could not identify health utilities in literature associated with cetuximab plus irinotecan for third-line therapy for mCRC, and generally the utility of patients treating with chemotherapy should be lower than that of BSC, so the study assumed these patients had the same health utility as BSC group participated in CONCUR trial with mean scores 0.75 at baseline and 0.57 upon disease progression. For all patients, the baseline health utilities were applied to stable state and health utilities documented at the end of treatment were applied to disease progression state until the terminal node (death). Throughout the model, the health utility was assumed invariant with respect to its underlying health state. While the occurrence of common severe adverse events (Grade 3/4) as reported in respective trials were included in the model (for cost estimate), no reduction in health utility (disutility) was explicitly incorporated in the model.
Cost Estimates
Only direct medical costs were considered and estimated from the perspective of third-party payer perspective in China, using 2017 Chinese RMB (¥). As such, the costs included both payment from third-party payers and patient out-of-pocket. The cost for regorafenib was calculated based on whole sale price, and cost for cetuximab plus irinotecan was calculated based on a cost survey in China. The study assumed all patients were treated with recommended dosing and administration schedules as approved in China product label, and patients remained on the treatment until disease progression or death, whichever came earlier.
To estimate non-anticancer drug related direct costs, the study convened an oncologist survey, which was composed of 18 oncologists working in tertiary hospitals from six metropolitan cities in China. Each oncologist was asked to complete a cost survey questionnaire for their most recent representative patients with mCRC treated with regorafenib or cetuximab plus irinotecan. The cost components included utilization of both in-hospital and outpatient care facilities, administration of medications via parenteral routes, use of supportive care medications, clinical monitoring with lab tests and diagnoses imaging, and care for treatment-emergent severe AEs. Frequencies of clinical followups and monitoring were based on current clinical practice reported by the panel rather than protocol driven schedules/ interventions as reported in the clinical trials. The survey questionnaire captured both frequencies of health care resource utilization and their respective unit cost. The median values were used for model input.
Base Case and Sensitivity Analyses
For each regimen of interest, estimates were made for expected LYs, QALYs, and overall direct costs. ICER of regorafenib vs. cetuximab plus irinotecan was presented in terms of the incremental cost per LY gained and per QALY gained as appropriate. The study presented both undiscounted and discounted values with a 3.5% annual discounting rate applied to all future benefits and costs.
To test whether the results of cost-effectiveness were robust to changes in assumptions and to address uncertainty in the estimation of model parameters in base case scenario, the study conducted a series of one-way sensitivity analyses on PFS, OS and drug costs for both regorafenib and cetuximab plus irinotecan regimen. Survival data and drug costs were varied with + 10% of their baseline values. In univariate sensitivity analysis, the study varied the value of one parameter at a time over its defined range examined the effect on the ICER. To assess the joint uncertainty associated with multiple parameters, the study performed probabilistic sensitivity analyses with the Monte Carlo simulations with 1,000 iterations, each time randomly sampling from the distribution for all parameters simultaneously. The study used gamma distribution for the cost parameters, exponential distribution for survival parameters, and triangle distribution for health utilities. The baseline values, ranges, and distributions of model parameters are listed in Table 1.
Table 1 The baseline values, ranges, and distributions of model parameters.
| Item | Regorafenib | Cetuximab plus irinotecan | Distribution |
|---|---|---|---|
| Efficacy: Full population | |||
| OS | 8.8 mon [7.9~9.7] | 8.6 mon [7.7~9.5] | Exponential |
| PFS | 3.2 mon [2.9~3.5] | 4.2 mon [3.8~4.6] | Exponential |
| Efficacy: Subpopulation | |||
| OS | 9.7 mon [8.7~10.7] | 8.6 mon [7.74~9.5] | |
| PFS | 5.4 mon [4.8~5.9] | 4.1 mon [3.7~4.5] | |
| Utility | |||
| PFS | 0.84 | 0.75 | Triangle |
| PD | 0.57 | 0.57 | Triangle |
| Incidence rate per week of adverse events | |||
| Hypertension | 0.68% | 0.00% | |
| Fatigue | 0.00% | 0.80% | |
| Hand-foot skin reaction | 1.03% | 0.00% | |
| Hyperbilirubinaemia | 0.40% | 0.00% | |
| Alanine aminotransferase concentration increased | 0.40% | 0.00% | |
| Neutropenia | 0.00% | 0.60% | |
| Rash | 0.00% | 0.60% | |
| Diarrhoea | 0.00% | 1.40% | |
| Cost per week | |||
| Oncology drug cost | ¥7,560.00 a | ¥11,083.38 b | Gamma |
| Anti-acid / anti-ulcer | ¥11.67 | ¥ 150.00 | |
| Anti-diarrheals | ¥- | ¥ 1.25 | |
| Anti-emetics | ¥33.33 | ¥ 150.00 | |
| NSAIDs | ¥- | ¥ 7.50 | |
| Opiates | ¥1.25 | ¥12.50 | |
| Other | ¥250.00 | ¥ 850 | |
| Inpatient | ¥57.50 | ¥ 212.5 | |
| Outpatient | ¥ 5.63 | ¥ 12.50 | |
| Management fee | ¥- | ¥ 87.5 | |
| Examination | ¥173.33 | ¥ 182.34 | |
| Test | ¥146.25 | ¥ 1,735.50 | |
| Cost per case | |||
| Hypertension | ¥ 600.00 | ||
| Fatigue | ¥- | ||
| Hand-foot skin reaction | ¥100.00 | ||
| Hyperbilirubinaemia | ¥ 675.00 | ||
| Alanine aminotransferase concentration increased | ¥600.00 | ||
| Neutropenia | ¥500.00 | ||
| Rash | ¥150.00 | ||
| Diarrhoea | ¥100.00 | ||
a. The price of regorafenib is 10,080 yuan (28 tablets for one week treatment) per box in China. Taking 3 weeks and stopping a week in each month, the weekly average drug cost of regorafenib is 7,560 yuan.
b. Patient assistance program (PAP) for cetuximab is included inthis model.
Results
Base case analysis
Discounted lifetime total treatment costs were lower for regorafenib at ¥221,860 compared with ¥402,218 for cetuximab plus irinotecan, resulting in cost-saving of ¥195,756 in lifetime total healthcare costs per patient treated. Most of the savings was explained by much lower drug cost associated with regorafenib vs. cetuximab plus irinotecan regimen (¥200,410 vs. ¥349,728) (Table 2). The results were robust with discounting or not.
Table 2 Base case results: cost-effectiveness outcome (average per patient) full population.
| Discounted | ||||
|---|---|---|---|---|
| Outcomes (Years) | Reg | Cet-Iri | Δ | |
| LY | 1.01 | 0.99 | 0.02 | |
| QALY | 0.68 | 0.65 | 0.03 | |
| Drug Cost | ¥200,410 | ¥349,728 | (¥149,318) | |
| AE Cost | ¥437 | ¥321 | ¥116 | |
| Inpatient Cost | ¥3,021 | ¥6,913 | (¥3,892) | |
| Outpatient Cost | ¥296 | ¥465 | (¥169) | |
| Other Direct Cost | ¥17,697 | ¥60,188 | (¥42,491) | |
| Total Cost | ¥221,860 | ¥417,616 | (¥195,756) | |
| ICER | - | - | - | |
| ICER QALY (Reg Vs Cet+Iri) | ICER | Dominant (=195,7560.03/¥) | ||
| ICER LY (Reg Vs Cet+Iri) | ICER | Dominant | ||
Reg: regorafenib; Cet+Iri: cetuximab+ irinotecan; LYs=Life-years; QALYs=Quality-adjusted life-years.
The base case scenario projected that, relative to cetuximab plus irinotecan regimen, regorafenib was in dominant position for both LYs and QALYs gained because of better health outcomes at lower total costs. The model projected patients on regorafenib had an incremental gain of 0.03 QALYs relative to cetuximab plus irinotecan (0.68 vs. 0.65) at a cost-saving of ¥195,756.
For the subpopulation who received no previous targeted treatment, compared to cetuximab plus irinotecan, regorafenib was expected to result in additional gains of 0.12 LYs and 0.15 QALYs at cost-saving of ¥95,987.
Analyses
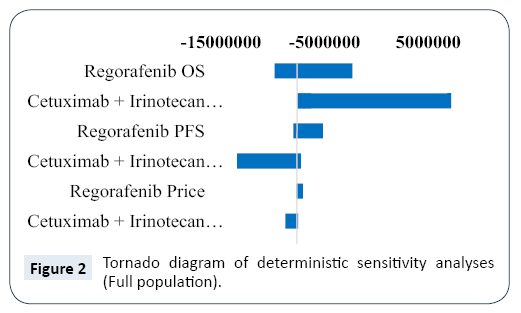
Results of deterministic sensitivity analyses are displayed in the tornado diagram in Figure 2. The model was most sensitive to change in OS parameter for cetuximab plus irinotecan. The changes in OS for regorafenib and PFS for cetuximab plus irinotecan within defined ranges had moderate impact, and PFS for regorafenib and prices for cetuximab plus irinotecan and regorafenib had little impact in the model output. Across broad variation in the ranges for each parameter, regorafenib was dominant relative to cetuximab plus irinotecan regimen with exception of the OS parameter for cetuximab plus irinotecan being at its upper value.
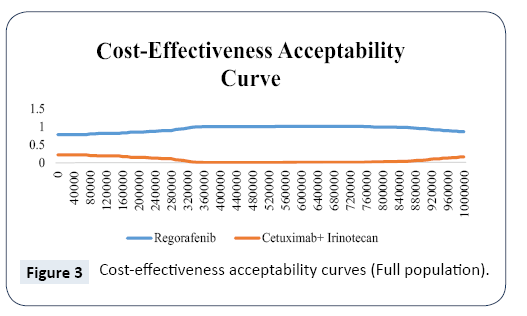
Results of probabilistic sensitivity analyses are shown in the cost-effectiveness acceptability curves in Figure 3. These curves indicate the probability that regorafenib is cost-effective across increasing willingness-to-pay (WTP) values per QALY gained. The results indicate that regorafenib has the probability of 82% to be cost-effective against with cetuximab plus irinotecan across the threshold range up to ¥161,980 (3 times of GDP per capita in 2016).
Discussion
Regorafenib has been licensed in US and European Union as a third-line option for the treatment of patients with mCRC who failed standard of care regimens. Both clinical practice guidelines developed by European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) also recommend regorafenib be considered as a third-line treatment option and for patients who have progressed through all available regimens [12,13]. In China, regorafenib was approved by CFDA for mCRC in March 2017. As regorafenib and cetuximab plus irinotecan have been recommended by diagnosis and treatment guideline of colorectal cancer in China (2017 Edition). Both regorafenib and cetuximab plus irinotecan regimen demonstrated survival benefits in controlled clinical trials. However, the introduction of novel anti-cancer drugs inevitably adds pressure to finite health care resources and health care policy makers need to be judicious in making decisions on optimal resource allocation. The study conducted this economic evaluation to add policy makers in their pricing and reimbursement decisions as well as to support oncologists in their clinical practice for later line treatment of mCRC.
The results from our analysis showed that regorafenib was dominant vis-a-vis cetuximab plus irinotecan regimen regarding LYs and QALYs gained in third-line treatment setting for mCRC in China, i.e., with better health outcomes at lower total costs. While it is uncommon to conclude one agent is dominant over another one in health economic evaluation, this does not come as a complete surprise as regorafenib-treated patients experienced slightly longer survival compared to their counterparts on cetuximab plus irinotecan regimen. And based on pricing information provided by regorafenib manufacturer, drug cost for regorafenib is less than half of cetuximab plus irinotecan regimen. Results from both univariate sensitivity analysis and probabilistic sensitivity analysis indicated that the model output appeared to be robust in response to changes to key variable values. For regorafenib reversing its dominant position, OS parameter for cetuximab plus irinotecan regimen had to be at its upper assumed value.
An earlier analysis of the cost-effectiveness of regorafenib as a third-line agent in patients with mCRC concluded that it was not cost effective in US setting with the ICERs being more than $550,000 per QALY gained in both base case and sensitivity analyses [14]. However, this analysis evaluated economic implications of regorafenib relative to placebo. The main thrust and applications of health economic evaluations are to compare alternative interventions competing for similar health care resources. Furthermore, conclusions of cost-effectiveness analysis conducted in one country does not necessarily apply in other countries as considerable variation in drug price, local treatment patterns, resources use and their unit cost, and different acceptance thresholds for cost-effectiveness.
Cetuximab plus irinotecan regimen had been subject to a few cost-effectiveness analyses to date and all of them compared this regimen with best supportive care or active supportive care [Starling: BJC; 2007] [15-17]. Norum conducted a model-based cost-effectiveness analysis for Norwegian patients and concluded that this regimen was not effective at cost per LY gained in the range between 205,536-323,040 Euros [16]. Annemanns et al estimated the ICERs for Cetuximab plus irinotecan regimen with fixed treatment duration of 6 weeks and 12 weeks in a Belgium setting and concluded the regimen was cost-effective in both scenarios [17]. Starling et al conducted both cost-effectiveness and cost-utility analyses with the ICERs being $79,073 per LY and $105,997 per QALY gained [15]. The divergent conclusions from these analyses illustrate the need to conduct health economic evaluation at local level.
To our knowledge, our analysis is the first cost-effectiveness analysis to evaluate regorafenib relative to another commonly used third-line treatment for mCRC. Ideally, evidence of clinical outcomes should be derived from a head-to-head controlled trial. In this analysis, the estimates of survival parameters were based on separate clinical trials owing to the absence of a direct head-to-head trial. The results in favor of regorafenib reported here, however, is unlikely to be biased significantly despite use of indirect clinical evidence from different trials. It is well documented that in metastatic cancer treatment setting, both clinical response to the treatment and duration of response would diminish with each additional line of treatment, reflecting refractory nature of advanced stage of cancer. In this context, 38% of patients enrolled in CONCUR trial received 4 or more previous systemic anticancer treatment lines after diagnosis of metastatic disease. In comparison, all patients receiving cetuximab plus irinotecan used in our analysis received no more than 3 prior chemotherapy regimens (74% had received only 2 prior chemotherapy regimens) [11].
There are two phase 3 pivotal trials with similar design in regorafenib clinical development programs for mCRC and both demonstrated survival benefits vs. placebo (plus best supportive care). The study opted to use the findings from CONCUR trial because 82% of the participants in this trial were Chinese and clinical benefits observed were more pertinent in this economic evaluation from perspective of local payers and clinicians. Survival data for cetuximab plus irinotecan regimen included in our analysis appears to be robust despite the trial was one-arm open label in design. Previously, another randomized control trial compared survival in irinotecan-refractory patients receiving cetuximab plus irinotecan regimen with cetuximab monotherapy [7]. The patients receiving cetuximab plus irinotecan regimen had nearly identical PFS and OS (4.1 and 8.6 months, respectively) in comparison to the survival data used in our analysis (4.2 and 8.6 months, respectively).
There are a few limitations that need to be acknowledged in our analysis. No health utility data were collected in the cetuximab plus irinotecan trial and the study could not identify utility values associated with cetuximab plus irinotecan therapy for mCRC in literature. As a result, the study assumed patients receiving cetuximab plus irinotecan regimen had the same utility values as those patients enrolled in the placebo group in CONCUR trial. Since patients in both groups in CONCUR trial reported the same utility value (0.57) at the end of treatment (mostly due to disease progression) and regorafenib-treated patients reported a higher health utility value at baseline vs. placebo group (0.84 vs. 0.75), the net effect was that regorafenib-treated patients were subject to greater utility decrement upon disease progression vs. patients receiving cetuximab plus irinotecan, i.e., in favor of cetuximab plus irinotecan for the QALY estimate. The study did not explicitly model utility penalty (disutility) in patients experiencing AEs of Grade 3 or 4 because precise disutility associated with each unique AE is not available or not reliable. In addition, changes in utility values from baseline to the end of treatment reflected patient’s overall utilities, which included potential disutility associated with treatment-emergent AEs. Finally, the study estimated effectiveness of anti-neoplasm from controlled trial, which may not reflect real world practice. Patients enrolled in clinical trials tended to be highly selected and had good functional status. Real-world effectiveness is likely to be worse and this would make the ICER higher. However, selection bias, if any in this case, was applied to both treatment groups equally and unlikely to change the results materially.
Conclusions
The therapeutic landscape of mCRC has evolved significantly over the last decide with the advent of new targeted therapy options. Third-party payers, including government payers, are increasingly utilizing health economic assessment as a tool in guiding their deliberation and decision making for optimal resource allocation. Our health economic evaluation shows the robust advantage of using regorafenib as a preferred agent versus cetuximab plus irinotecan regimen in treatment patients with mCRC at third-line treatment setting; more LYs and QALYs gains at lower total costs.
References
- Globocan (2012) colorectal cancer fact sheet 2012.
- Wanqing Chen, Rongshou Zheng, Peter D, Baade, Siwei Zhang, et al. (2015) Cancer Statistics in China. CA Cancer J Clin 2016.
- Lim HJ, Gill S, Speers C, Barbara Melosky, Jeff Barnett, et al. (2009) Impact of irinotecan and oxaliplatin on overall survival in patients with metastatic colorectal cancer: a population-based study. J Oncol Pract 5: 153-158.
- Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, et al. (2008) Urgent Need for a New Staging System in Advanced Colorectal Cancer. J Clin Oncol 26: 4828-4833.
- Welch S, Spithoff K, Rumble RB, Maroun J (2010) Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol 21: 1152-62.
- Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Dongsheng Tu MD, et al. (2007) Cetuximab for the Treatment of Colorectal Cancer. N Engl J Med 357: 2040-2048.
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, et al. (2004) Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer. N Engl J Med; 351: 337-345.
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA ,et al. (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129: 245-255.
- Li J, Qin S, Xu R, Ruihua Xu, MD, Thomas C, Brigette Ma, et al. (2015) Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol16: 619-629.
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, et al. (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381: 303-312.
- Spindler KL, Pallisgaard N, Lindebjerg J, Frifeldt SK, Jakobsen A (2011) EGFR related mutational status and association to clinical outcome of third-line cetuximab-irinotecan in metastatic colorectal cancer. BMC Cancer; 11:107.
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken, et al. (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27: 1386-422.
- NCCN. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf; accessed Dec 15, 2017
- Goldstein DA, Ahmad BB, Chen Q, Ayer T, Howard DH, et al. (2015) Cost-Effectiveness Analysis of Regorafenib for Metastatic Colorectal Cancer. J Clin Oncol 33: 3727-32.
- Starling N, Tilden D, White J, Cunningham D (2007) Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer 96: 206-212.
- Norum J (2006) Cetuximab in the treatment of metastatic colorectal cancer: a model-based cost-effectiveness analysis. J Chemother 18: 532-537.
- Annemans L, Van Cutsem E, Humblet Y, Van Laethem JL, Bleiberg H (2007) Cost-effectiveness of cetuximab in combination with irinotecan compared with current care in metastatic colorectal cancer after failure on irinotecan--a Belgian analysis. Acta Clin Belg 62: 419-425.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences