Projected Cost-Effectiveness of Liraglutide, a Glucagon-Like Insulin Peptide-1 Analogue in Comparison to Insulin Glargine in Type 2 Diabetes Patients with Suboptimal Glycaemic Control in Malaysia
Kamaruddin NA, Bidin MBL, Tan SC, Shafie AA
Kamaruddin NA1, Bidin MBL2, Tan SC3* and Shafie AA4
1Department of Medicine, Faculty of Medicine, National University of Malaysia, Kuala Lumpur, Malaysia
2Department of Medicine, Kuala Lumpur Hospital, Kuala Lumpur, Malaysia
3Health Economics and Outcomes Research, IMS Health Asia Pacific, Singapore
4School of Pharmaceutical Sciences, Universiti Sains Malaysia, Kota Baru, Malaysia
- Corresponding Author:
- Tan SC Health
Economics and Outcomes Research
IMS Health Asia Pacific, Singapore
Tel: 65 6412 7310
E-mail: sctan@sg.imshealth.com
Received Date: March 18, 2016, Accepted Date: May 7, 2016, Published Date: May 17, 2016
Copyright: © 2016 Kamaruddin NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: The objective of this study was to evaluate the long-term cost-effectiveness of liraglutide versus insulin glargine in Malaysia for obese patients with uncontrolled T2DM patients receiving 2-3 oral anti-diabetics (OADs).
Methods: The perspective of this analysis was societal. Cost-effecitveness was simulated using the validated IMS CORE Diabetes Model, with a time horizon of 40 years. Baseline characteristics of patients and treatment effectiveness was derived from LEAD5, a head-to-head trial comparing liraglutide and glargine. Published local cost data and resource use inputs were used. All costs were reported in 2014 Malaysian Ringgit (MYR). A 3% discount rate was applied. One-way and probabilistic sensitivity analyses were conducted to test the robustness of the results.
Results: The base case analysis result found that treatment with liraglutide in comparison to glargine was associated with a gain of 0.216 quality-adjusted life years (QALY) and an incremental cost of MYR12,132, resulting in an incremental cost-effectiveness ratio of MYR56,120 per QALY gained. Sensitivity analyses indicated the result wass sensitive to changes in parameters in particular number of treatment years and daily dose of liraglutide. However, none of the sensitivity analyses resulted in an ICER above the WHO’s recommended threshold of 3 times GDP per capita of Malaysia in 2014.
Conclusion: Treating poorly controlled obese T2DM patients in Malaysia with liraglutide instead of insulin glargine for an initial treatment period of up to 5 years was projected to be a cost-effective strategy resulting in beneficial outcomes, including lower rates of long-tem complications and higher quality-adjusted life expectancy.
Keywords
Liraglutide; Insulin glargine; Costeffectiveness; Malaysia
Introduction
Diabetes is a common chronic disease associated with significant morbidity and mortality. The life expectancy of patients with diabetes is reduced by up to ten years compared to the general population, mainly due to the increased risk of cardiovascular death and stroke [1]. Research estimated that 60-90% of all T2DM cases being related to obesity [2]. Diabetes-related complications accounts for the majority of T2DM direct medical costs [3].
The clinical goal in the treatment of diabetes is to achieve good glycaemic control, measured by the glycated haemoglobin (HbA1c) level. It is well established that improving glycaemic control and other cardiovascular risk factors can improve health-related quality of life (HRQoL) [4], and significantly reduce overall healthcare costs [5].
Empirical evidence suggests that T2DM poses a significant public health challenge to the Malaysian government. The most recent National Health and Morbidity Survey (NHMS) conducted by the Malaysia Ministry of Health (MmoH) indicates a prevalence of T2DM of 15.2% in Malaysia [6]. Furthermore, overall diabetes prevalence in 2013 was 22.6% and more than half (53%) of people with diabetes remain undiagnosed [7]. Even among the diagnosed population, only 22% were estimated to have achieved the treatment goal of HbA1c level < 7.0% [8].
Liraglutide, a glucagon-like insulin peptide-1 (GLP-1) analogue, has demonstrated efficacy and safety for the treatment of type 2 diabetes mellitus (T2DM) in obese adults, when used in combination with oral anti-diabetic medications (OADs). The daily recommended dose is 1.2 mg to 1.8 mg. In our model, a mean dose of 1.3 mg once daily was utilized based on real world data from a Malaysian clinical audit of 164 patients [9].
The extensive phase 3 Liraglutide Effect and Action in Diabetes (LEAD) clinical trial programme demonstrated superior efficacy and safety of liraglutide for reducing HbA1c, minimizing weight gain and other risk factors compared with other OADs [10]. A recent systematic review found liraglutide to be a cost-effective adjunct treatment for T2DM, which may also be associated with a reduction in diabetes-related complications costs [11].
The present analysis was conducted from the societal perspective in Malaysia. The objective of this analysis was to evaluate the long-term cost-effectiveness of liraglutide as an adjunct therapy for a hypothetical cohort of T2DM patients with poorly controlled diabetes (HbA1c ≥ 7% and ≤ 10%) (despite receiving current combination of 2 or more OADs), obese (BMI ≥ 32 kg/m2), and either high risk of hypoglycemia or established cardiovascular disease.
Methods
Study design
A validated simulation model, the IMS CORE Diabetes Model, was utilized to evaluate the long-term costeffectiveness of liraglutide for the treatment of obese T2DM patients in Malaysia. The choice of comparator was based on the rationale that there is currently no GLP-1 in the Malaysian MoH Drug Formulary and such patients were treated with basal insulin add-on to OADs. Insulin glargine, a listed analogue insulin which is less likely to cause hypoglycaemia compared to human basal insulin [12], is the comparator selected for this analysis.
The model efficacy parameter inputs were based on the published study manuscript of LEAD 5 while local literature and reports were referenced for unit cost and resource use.
Incremental cost-effectiveness ratios (ICERs) per qualityadjusted life year gained were calculated for liraglutide versus insulin glargine. The base case analyses were conducted from a societal perspective, which captured treatment acquisition costs including other direct medical costs and productivity loss (days off work) due to diabetes-related complications.
A series of one-way sensitivity analyses and probabilistic sensitivity analysis (PSA) were conducted to test the robustness of the cost-effectiveness results to plausible changes in the model parameters.
Simulation model
The IMS CORE Diabetes Model (CDM) is a computer simulation model that was developed to determine the longterm health effects and cost consequences of interventions in type 1 (T1DM) and type 2 diabetes mellitus (T2DM). The model is accessible on a licensed basis over the internet. A detailed explanation of the CDM could be found in publications such as Palmer et al. [13]. In brief, IMS CORE is a computer simulation model designed to assess the lifetime health outcomes and costs of interventions in type 1 or type 2 diabetes mellitus. The model structure comprises 17 interdependent underlying models that simulate the complications of diabetes, including angina pectoris, myocardial infarction (MI), congestive heart failure (CHF), stroke, peripheral vascular disease, diabetic retinopathy, macular edema, cataracts, hypoglycemia, ketoacidosis, nephropathy, neuropathy, foot ulcer and amputation, pulmonary edema, and depression, in addition to nonspecific mortality. The model allows the calculation of both direct and indirect costs; adjusted for quality of life and can be utilized to perform cost-effectiveness and cost-utility analyses.
Patient population
The baseline cohort for the simulation model was defined according to data from LEAD5, which was a randomized controlled trial of 581 patients with type 2 diabetes from 17 countries, [14] LEAD5 was part of the Phase 3 Liraglutide Effect and Action in Diabetes (LEAD) clinical trial programme involving more than 6,000 patients recruited from over 600 sites in 40 countries. Liraglutide and insulin glargine are both once-daily insulins, administered by subcutaneous injection in the abdomen, thigh or upper arm using a pre-filled pen device administered at any time during the day. For more information on titration algorithm and drug administration during the trial can be found elsewhere [14].
Given the LEAD5 study is the only head-to-head study comparing liraglutide against glargine, we conservatively assumed the population of interest for this analysis would resemble an “average” trial participants in the LEAD5 study [15]. While other characteristics were assumed to be identical, the average BMI of the population was modified to 32 kg/m2, for modelling on obese Malaysian T2DM patients targeted for treatment by liraglutide (Table 1).
Table 1: Baseline characteristics and complication rates – LEAD5.
| Characteristic | Value |
|---|---|
| HbA1c (%) | 8.2 |
| Age (years) | 57.7 |
| Male (proportion) | 0.565 |
| Duration of diabetes (years) | 9.00 |
| Systolic blood pressure (mmHg) | 134.00 |
| Baseline total cholesterol (mg/dL) | 185.30 |
| Baseline HDL-C (mg/dL) | 50.20 |
| Baseline LDL-C (mg/dL) | 119.70 |
| Baseline triglycerides (mg/dL) | 194.70 |
| BMI (kg/m2)* | 32.00 |
| Proportion smoker | 0.20 |
| Cigarettes per day | 10 |
| Alcohol consumption (Oz per week) | 5 |
BMI: body mass index, HbA1c: glycatedhaemoglobin, HDL: high-density lipoprotein, LDL: low-density lipoprotein
Intervention and comparator
As T2DM symptoms typically worsen over time, it is likely that patients on liraglutide will need to intensify their treatment regime at some point, and thus would not remain on liraglutide for the entire duration of the current analysis. Based on this assumption, at the commencement of the simulation, patients were assigned to receive either treatment with liraglutide 1.8 mg or with insulin glargine. We assumed patients starting on liraglutide 1.8 mg would remain on treatment for 3 years. At year 4, all patients in the liraglutide arm were assumed to switch to the insulin glargine regimen. This approach is similar to the treatment pathway assumptions considered for patients treated with liraglutide in the UK NICE final appraisal determination [16].
Treatment effects
Treatment effects associated with liraglutide versus glargine were drawn from the LEAD5 study [15] (Table 2). Changes in HbA1c, SBP, total cholesterol (TC), high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol and triglycerides were simulated along with changes in BMI and hypoglycaemic events. Both liraglutide and glargine were shown to decrease HbA1c levels from baseline (-1.33% and -1.09%, respectively). BMI decreased in the liraglutide and increased in the glargine treatment arms ( 0.644 kg/m2 and 0.577 kg/m2, respectively).
Table 2: Clinical inputs based on LEAD5 results.
| Parameter | Mean change from baseline ± SE | |
|---|---|---|
| Liraglutide 1.8mg | Glargine | |
| HbA1c (%) | -1.33±0.09 | -1.09±0.09 |
| Systolic blood pressure (mmHg) | -3.79±19.61 | 0.54±20.35 |
| Total cholesterol (mg/dL) | -2.36±41.31 | 2.77±42.68 |
| HDL-cholesterol (mg/dL) | -2.32±9.58 | -2.07±9.94 |
| LDL-cholesterol (mg/dL) | 4.19±35.17 | 9.15±36.25 |
| Triglycerides (mg/dL) | -21.79±149.22 | 19.52±153.89 |
| BMI (kg/m2) | -0.64±1.37 | 0.58±1.50 |
| Major hypoglycaemia* (events/100 patient years) | 3 | 3 |
| Minor hypoglycaemia* (events/100 patient years) | 125 | 125 |
BMI: body mass index, HbA1c: glycated haemoglobin, HDL: high-density lipoprotein, LDL: low-density lipoprotein, SE: standard error
*Given similar low rates with no significant difference were reported for hypoglycaemia in LEAD5, we assumed both treatment groups have the same risks of minor and major hypoglycaemia by using the corresponding overall average rates reported in LEAD5.
Costs and perspective
The base case adopted the societal perspective with both direct and indirect costs were included. The annual diabetes therapy cost, clinical consultation fees and costs of treating complications were derived from published local literature [17-19] and internal analyses at selected tertiary hospitals in Malaysia (Appendix 1). The published costs were inflated with an annual rate of 5% to estimate costs in 2014. A series of structured interviews with a panel of local experts verified the model structure, assumptions, resource use and cost data that was identified and used in the model.
For the calculation of indirect costs resulting from a loss of productivity, average annual wages of MYR 25,216 for males and MYR 24,317 for females in 2014 were assumed by applying 5% annual inflation rates on the reported 2012 estimates [20], with a working year consisting of an average of 260 days.
Health outcomes
For BMI progression, the respective weight change estimates were applied for respective interventions at the beginning of the simulations, and then it was assumed that patients regained their baseline weight at the initiation of insulin. It is worth noting that the switch to insulin glargine at the end of the initial treatment period results in an initial expected weight gain (as for insulin glargine in the LEAD5 trial) which was applied across both interventions.
For T2DM and its complications, uhealth utility inputs for the model were derived primarily from the UKPDS [21] supplemented with other data sources as necessary [22-28].
Disutility for excess BMI is built into the IMS CORE Diabetes Model for base case analysis. Weight is an important factor in T2DM and it is well established that different treatments for T2DM have different effects on weight.
The utility data applied are based on a published equation by Bagust et al. evaluating time trade-off scores based on 4,612 patients with T2DM [5] that completed the EQ-5D questionnaire (CODE-2 study). The equation is based upon complication presence in the area of stroke, nephropathy, neuropathy, peripheral vascular disease, foot ulceration, amputation and eye disease. Included patient data are age, gender, duration of diabetes, BMI, and treatment type (oral anti-diabetics or insulin). Each BMI unit increase of 1 kg/m2 above a value of 25 kg/m2 reduces the patient utility score by a value of 0.0061.
Time horizon and discounting
A lifetime horizon (40 years or until death) was used in this analysis. A lifetime perspective was taken as T2DM is a chronic disease that has health and cost implications for patients over the long-term due to diabetes-related complications, morbidity and mortality. Costs and outcomes were both discounted at 3% annually [14].
Sensitivity analyses
Multiple sensitivity analyses were conducted to evaluate how the uncertainty surrounding the input parameters, such as time horizon, discount rate, clinical benefits and complication costs, affect the incremental cost-effectiveness results. Probabilistic sensitivity analysis (PSA) was conducted to test the robustness of the base-case result by simultaneously varying the values of all input parameters, which were sampled from assigned distributions, to obtain 1,000 estimates of the incremental cost and effectiveness, the mean of which was used to generate cost-effectiveness acceptability curve (CEAC).
Results
Base case analysis
The base case deterministic results demonstrate that the treatment with liraglutide was associated with a greater quality-adjusted life year (QALY) than glargine (8.260 vs. 8.044), which outweighed the difference in combined costs (MYR 132,545 vs. MYR 120,413), leading to incremental costeffectiveness ratio for liraglutide compared to insulin glargine which was estimated to be at MYR 56,120 per QALY (Table 3). The WHO’s recommended threshold for suggesting a treatment to be cost-effective is for the ICER value to be less than 3 times of the projected GDP per capita [29]. In the case of Malaysia in 2014, this threshold works out to be MYR107,817 (3 × MYR35,939), and therefore liraglutide is considered more cost-effective than glargine for the target population in the local context of Malaysia.
Table 3: Base-case deterministic results.
| Liraglutide 1.8mg | Insulin Glargine | |
|---|---|---|
| QALY | 8.26 | 8.044 |
| Direct Costs (MYR) | 126,260 | 114,069 |
| Indirect Costs (MYR) | 6,285 | 6,344 |
| Combined Costs | 132,545 | 120,413 |
| ICUR (MYR per QALY gained): Liraglutide vs. Glargine |
56,120 | |
ICUR: incremental cost-utility ratio, QALY: quality-adjusted life-year
Liraglutide was also found to have lower rates of diabetes complications than insulin glargine, including eye disease, renal disease, ulcer, and cardiovascular disease (Table 4).
Table 4: Treatment costs.
| Description | Annual Cost (MYR) | Source |
|---|---|---|
| Drug costs | ||
| Liraglutide 1.8 mg daily | 7,938.75 | Data on File |
| Liraglutide 1.2 mg daily | 5,292.50 | Data on File |
| Insulin glargine 24 IU daily | 1,068.72 | Data on File |
| Insulin glargine 50 IU daily | 2,226.50 | Data on File |
| Clinical consultation costs | ||
| 1st year | 300 | Hospital internal analysis |
| 2nd year onwards | 200 | Hospital internal analysis |
| Management costs | ||
| Statins | 480 | Hospital internal analysis |
| Aspirin | 120 | Hospital internal analysis |
| ACE-I | 110 | Hospital internal analysis |
| Anti-depression | 110 | Hospital internal analysis |
| Screening eye | 75 | Hospital internal analysis |
| Screening for MA | 45 | Hospital internal analysis |
| Direct costs - cardiovascular complications | ||
| Myocardial infarction 1st year | 36,300 | [30-31] |
| Myocardial infarction 2nd+ years | 28,500 | Hospital internal analysis |
| Angina 1st year | 12,400 | Hospital internal analysis |
| Angina 2nd+ years | 11,400 | Hospital internal analysis |
| Congestive heart failure 1st year | 16,450 | Hospital internal analysis |
| Congestive heart failure 2nd+ years | 13,200 | Hospital internal analysis |
| Stroke 1st year | 14,800 | Hospital internal analysis |
| Stroke 2nd+ years | 10,800 | Hospital internal analysis |
| Stroke death within 30 days | 4,000 | Hospital internal analysis |
| Peripheral vascular disease 1st year | 25,400 | [32] |
| Peripheral vascular disease 2nd+ years | 7,200 | Hospital internal analysis |
| Direct costs - renal complications | ||
| Haemodialysis 1st year | 47,782 | [54] |
| Haemodialysis 2+ years | 47,782 | [54] |
| Peritoneal Dialysis 1st year | 46,776 | [54] |
| Peritoneal Dialysis 2+ years | 46,776 | [54] |
| Renal transplant costs 1st year | 115,000 | Hospital internal analysis |
| Renal transplant 2+ years | 30,000 | Hospital internal analysis |
| Direct costs – acute events | ||
| Major hypoglycaemia (per event) | 3,881 | [32] |
| Minor hypoglycaemia (per event) | 0 | Hospital internal analysis |
| Direct costs – eye disease | ||
| Laser treatment | 725 | Hospital internal analysis |
| Cataract operation | 1,250 | Hospital internal analysis |
| Cost following cataract operation | 275 | Hospital internal analysis |
| Cost blindness - year of onset | 2,225 | Hospital internal analysis |
| Cost blindness - following years | 300 | Hospital internal analysis |
| Direct costs – neuropathy, foot ulcer, amputation | ||
| Neuropathy 1st year | 800 | Hospital internal analysis |
| Neuropathy 2nd+ years | 300 | Hospital internal analysis |
| Amputation (event based) | 1,425 | Hospital internal analysis |
| Amputation prosthesis (event based) | 2,850 | Hospital internal analysis |
| Gangrene treatment (monthly) | 2,200 | Hospital internal analysis |
| Healed ulcer | 250 | Hospital internal analysis |
| Infected ulcer (monthly) | 1,875 | Hospital internal analysis |
| Standard uninfected ulcer (monthly) | 750 | Hospital internal analysis |
Sensitivity analyses
The ICER of liraglutide vs. insulin glargine was also found to be sensitive to variations in parameters such as dose of liraglutide (MYR22,085 for liraglutide 1.2 mg; MYR27,717 for liragltuide 1.3 mg), cost of liraglutide (MYR45,906 for -10%, MYR35,697 for -20%), application of different utilities as body weight changes (MYR71,830 for no utility impact of weight changes), baseline BMI (MYR 53,990 for BMI = 30; MYR 49,085 for BMI = 35), analysis time horizon (MYR140,140 for 10 years, MYR62,794 for 20 years), and assumed discount rates (MYR33,989 for 0%, MYR72,856 for 5%, MYR81,659 for 6%) (Table 5). The highest ICER of MYR 99,661 was observed for a liraglutide treatment duration of 5 years. As such, liraglutide was projected to remain cost-effective compared to glargine in all sensitivity scenarios, as per WHO’s recommendation.
Table 5: Cost-effectiveness results of one-way sensitivity analyses.
| Scenario | ICUR (MYR per QALYgained) |
|---|---|
| Base case | 56,120 |
| Baseline BMI = 35kg/m2 | 49,085 |
| Liraglutide dose 1.2mg | 22,085 |
| Glargine dose 40-60IU/day | 50,458 |
| Liraglutide treatment duration | |
| 1 year | 4,897 |
| 5 years | 99,661 |
| Effects on BMI apply only during initial treatment period | 70,212 |
| Model time horizon | |
| 20 years | 62,794 |
| HbA1c efficacy | |
| Liraglutide 20% relative improvement | 47,743 |
| Glargine 20% relative improvement | 64,312 |
| Discount rates | |
| 0% | 33,989 |
| 5% | 72,856 |
| 6% | 81,659 |
| Complication costs | |
| -20% | 60,021 |
| +20% | 52,209 |
| Cost of liraglutide | |
| -10% | 45,906 |
| -20% | 35,697 |
| Weight: no direct utility impact | 71,830 |
| Hypoglycaemia disutility | |
| -0.0052 | 56,193 |
| None | 55,986 |
BMI: body mass index, HbA1c: glycated haemoglobin, ICUR: incremental cost-utility ratio, QALY: quality-adjusted life-year
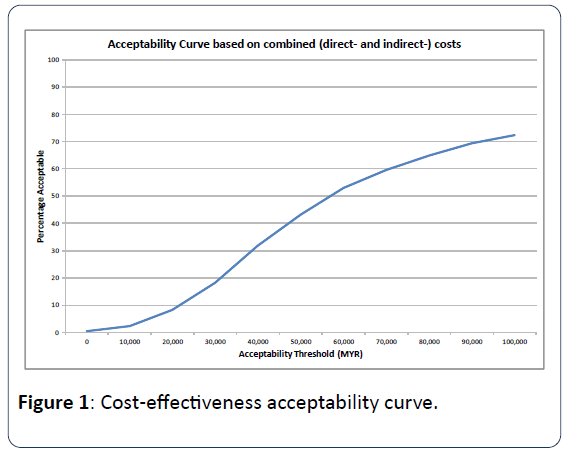
Taking the uncertainties of all input parameters jointly, the PSA (Probabilistic sensitivity analysis)) indicates that liraglutide 1.8 mg followed by insulin glargine was 18.2%, 59.6%, and 72.3% likely to be considered cost-effective at different thresholds of 30,000 MYR, 70,000 MYR, and 100,000 MYR, respectively (Figure 1).
Discussion
Based on a simulation of long-term cost-effectiveness of liraglutide compared to insulin glargine, the present analysis suggests that from a societal perspective, liraglutide is a more cost-effective option than glargine in managing obese patients in a Malaysian setting with uncontrolled diabetes despite 2-3 OADs with either high risk of hypoglycemia or established cardiovascular disease. Cost-effectiveness was evaluated based on the WHO’s recommended threshold of 3 times GDP per capita of Malaysia in 2014. By using the mean dose of 1.3 mg daily reported in a local clinical audit, the ICER of liraglutide against glargine was MYR27,717 which was well below 1 GDP per capita (MYR35,939) of Malaysia in 2014. Thiscould be interpreted that liraglutide is estimated to be highly more cost effective than glargine in the real world clinical setting in Malaysia. These findings may be useful to decisionmakers in the local context of Malaysia, particularly in addressing the unmet needs of obese T2DM patients with poorly controlled symptoms in Malaysia.
In the current analysis, patients treated with liraglutide were generally estimated to have lower cumulative incidence rates of diabetes-related complications than those treated with glargine, including eye disease, renal disease, ulcers, and cardiovascular disease.
Sensitivity analysis found that cost-effectiveness results of liraglutide as an add-on therapy when compared to glargine were robust to variations in treatment dose, treatment cost, initial treatment period, effect of body weight changes, discount rate and analysis time horizon. At a baseline BMI=30, similar cost-effectiveness result was observed with ICER of MYR 53,990, well below the threshold recommended by WHO. A separate sensitivity analysis was conducted to address the likely impact of uncertainty of complication costs which were not widely published in the local context of Malaysia. However, an ICER similar to that in base case was observed (MYR 60,021 vs. MYR 56,120), further confirming the robustness of the cost-effectiveness conclusion of liraglutide against glargine for the sub-population of interest in this evaluation.
A previous study conducted in China found liraglutide to be a cost-effective treatment approach compared to insulin glargine for treating T2DM in the short-term [30]. A health technology assessment produced by NICE also noted liraglutide to be cost-effective as an add-on therapy when compared to insulin glargine in a UK cohort based on a review on the evidence submitted by the manufacturer [31]. The current analysis builds on the literature by demonstrating the long-term cost-effectiveness of liraglutide as an adjunct therapy as compared to insulin glargine.
The observed cost-effectiveness, including higher clinical efficacy and lower incidence of diabetes-related complications, may be attributed to the better treatment efficacy profile observed in LEAD5 for liraglutide than insulin glargine [15]. Results from the trial found that liraglutide reduced HbA1c significantly vs. glargine (1.33% vs. 1.09%; -0.24% difference; p = 0.0015). There was also greater weight loss with liraglutide vs. glargine (treatment difference -3.43 kg; p < 0.0001) and greater reduction of systolic BP (-4.0 mmHg) vs. glargine (+0.5 mmHg; -4.5 mmHg difference; p = 0.0001). This means that part of the higher acquisition cost of liraglutide was predicted to be offset by the savings resulted from greater clinical efficacy and lower projected complication risks in the simulation through CDM.
Treatment with liraglutide is associated with weight loss in the literature, with a clinical effectiveness study finding that absolute body weight for T2DM patients treated with liraglutide decreased by 1.5 to 4.0 kg across four BMI categories, with greater weight loss occurring in higher BMI individuals [32]. A study on the economic implications of weight changes in T2DM patients found that a > 3% loss in weight was associated with statistically significant decreases in all-cause and T2DM-specific costs due to reduced utilization of medical services [33]. This explains the potential cost-offset attributed to relatively lower complication risk predicted for patients treated with liraglutide compared to those treated with glargine as a result of weight loss benefit from liraglutide treatment, compared to the weight gain experienced during treatment with glargine.
The comparative cost-effectiveness of liraglutide may also be attributed to the gain in QALE as a result of a better lipidlowering profile when compared to insulin glargine. Although the clinical inputs in this study were derived from the data collected in a phase 3 intervention trial, similar clinical evidence of liraglutide was observed in a real-life cohort liraglutide, further reinforcing the findings in the trials under LEAD clinical programme.
It is important to note that the target population of evaluation interest in this study tends to have more severe profile (uncontrolled diabetes despite 2-3 OADs, higher baseline BMI, risk/ history of recurrent hypoglycemia and cardiovascular comorbidities) than those in source trial (LEAD5). Despite this conservative approach, liraglutide was concluded to be more cost-effective than glargine in both base case scenarios and a series of sensitivity analyses in the Malaysian context by using the CORE Diabetes Model, which has been extensively published and validated against real-life data [34]. The CORE Diabetes Model uses complication risk equations derived from predominately Caucasian cohorts, however the model has previously been validated for, and utlized for Asian populations [34]. As such, we believe the study conclusion is valid and robust, since the consistent findings of liraglutide that was more cost-effective than insulin glargine in the local context of Malaysia was demonstrated through extensive sensitivity analyses.
Conclusion
Based on the IMS Core Diabetes Model Analysis, treatment of poorly controled obese T2DM patients with liraglutide instead of insulin glargine would be cost-effective, resulting in beneficial health outcomes including lower rates of long-term diabetes-related complications and higher quality-adjusted life expectancy.
Aknowledgement.
Authors’ contributions
All the authors contributed extensively to the work presented in this paper. Kamaruddin NA and Long Bidin MB led the study conception and adaptation to the local clinical practice with Shafie AA and Tan SC who conducted literature review, designed and implemented local data collection and analysis. All authors jointly contributed to result interpretations and manuscript writing. All authors read and approved the final manuscript.
Funding Sources
This study has been funded by Novo Nordisk including all costs associated with the development and the publishing of the present manuscript. Funding was not contingent upon publication of the manuscript.
Conflict of interest statement
Kamaruddin NA has served as a speaker, an advisory board member and a study investigator for GlaxoSmithKline, MSD, Novo Nordisk, and Sanofi-Aventis. Long Bidin MB has served as a study investigator or a speaker for Elli Lilly, MSD, Novartis, Merck Serono and Novo Nordisk. Shafie AA has served as as a speaker, an advisory board member and a study investigator for Astra Zeneca, Bayer, MSD, Pfizer, Novo Nordisk, Novartis, Kotra, and CCM. Tan SC is a current employee of IMS Health Asia Pacific, a consultancy that has received funds from Novo Nordisk. All the other authors declare that they have no competing interests.
References
- Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM (2001) Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ 322:1389-1393.
- Anderson JW, Kendall CW, Jenkins DJ (2003) Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am CollNutr 22:331-339.
- American Diabetes Association (2008) Economic costs of diabetes in the U.S. In 2007. Diabetes Care 31:596-615.
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, et al. (2003) Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348: 383-393.
- Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, et al. (2001) Effect of improved glycemic control on health care costs and utilization. JAMA 285: 182-189.
- Institute for Public Health (2011) The Fourth National Health and Morbidity Survey (NHMS IV), Nutritional Status. Ministry of Health, Malaysia.
- WanNWM, Md Isa SH, Wan Mohamad WB, Khir AS, Kamaruddin NA,et al. (2013) Prevalence of diabetes in Malaysia and usefulness of HbA1c as a diagnostic criterion. Diabet Med 30:825-828.
- Mafauzy M, Hussein Z, Chan SP (2011) The status of diabetes control in Malaysia: results of DiabCare2008. Med J Malaysia 66: 175-181.
- Kamaruddin NA, Mumtaz M, Chan SP, Hew FL (2014) Effect of adding Liraglutide to other anti-diabetic medications in achieving glycemic control in patients with type 2 diabetes mellitus.
- Jeong KH, Yoo BK(2011)The efficacy and safety of liraglutide. Int J Clin Pharm33: 740-749.
- Zueger PM, Schultz NM, Lee TA (2014)Cost effectiveness of liraglutide in type II diabetes: a systematic review. Pharmacoeconomics 32: 1079-1091.
- Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, et al. (2001) Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 24: 631-636.
- Palmer AJ, Roze S, Valentine WJ(2004) The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin20:S5-26.
- Pharmaceutical Services Division (2012) Pharmacoeconomic guideline for Malaysia.
- Russell-Jones D, Vaag A, Schmitz O (2009)Liraglutidevs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia52: 2046-2055.
- NICE (2010) Final appraisal determination: Liraglutide for the treatment of type 2 diabetes mellitus. UK.
- Aniza I, Syafrawati, Saperi S, Zafar M, Amrizal M, et al.(2011) Developing the cost for uncomplicated scutest elevated myocardial infarction (Stemi primary percutaneous coronary intervention) using step down and activity based costing at UKMMC. Journal KesihatanMasyarakat17: 26-31.
- Chin SP, Jeyaindran S, Azhari R(2008) Acute coronary syndrome (ACS) registry-leading the charge for National Cardiovascular Disease (NCVD) Database. Med J Malaysia 63: 29-36.
- Ibrahim W, Aljunid S, Ismail A (2010) Cost of type 2 diabetes mellitus in selected developing countries. Malaysian Journal of Public Health Medicine 10: 68-71.
- Statistics Office, Government of Malaysia (2012) Salaries and wages survey report 2012.
- Clarke P, Gray A, Holman R (2002) Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 22: 340-349.
- Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, et al. (2006) Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 22: 1523-1534.
- Lansingh VC, Carter MJ (2009) Use of global visual acuity data in a time trade-off approach to calculate the cost utility of cataract surgery. Arch Ophthalmol 127: 1183-1193.
- Lloyd A, Nafees B, Gavriel S, Rousculp MD, Boye KS, et al. (2008)Health utility values associated with diabetic retinopathy. Diabet Med 25: 618-624.
- Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, et al. (2004) Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab 30: 549-556.
- Sharma S, Oliver-Fernandez A, Bakal J, Hollands H, Brown GC, et al. (2003) Utilities associated with diabetic retinopathy: results from a Canadian sample. Br J Ophthalmol 87: 259-261.
- Wasserfallen JB, Halabi G, Saudan P(2004) Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant 19: 1594-1599.
- WHO (2003) Guide to cost-effectiveness analyses.
- Fan CS, Zhong J, Sun F,Liu F (2013) Evaluating short-term cost-effectiveness of liraglutide versus insulin glargine in patients with Type-2 diabetes in a chinese setting. Value in Health 16: A162-A163.
- Shyangdan D, Cummins E, Royle P, Waugh N (2011)Liraglutide for the treatment of type 2 diabetes. Health Technol Assess 15: 77-86.
- Chitnis AS, Ganz ML, Benjamin N, Langer J, Hammer M (2014) Clinical effectiveness of liraglutide across body mass index in patients with type 2 diabetes in the United States: a retrospective cohort study. AdvTher 31: 986-999.
- Bell K, Parasuraman S, Shah M (2014) Economic implications of weight change in patients with type 2 diabetes mellitus. Am J Manag Care 20: e320-329.
- McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A (2014) Validation of the IMS CORE Diabetes Model. Value Health. 17: 714-724.
- Chirakup S, Chaiyakunapruk N, Chaikledkeaw U (2008) Cost-effectiveness analysis of thiazolidinediones in uncontrolled type 2 diabetic patients receiving sulfonylureas and metformin in Thailand. Value Health1:S43-S51.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences