Adherence to Lurasidone and other Atypical Antipsychotics among Patients with Bipolar Disorder: A Real World Assessment
Rajagopalan K, Wade SW, Chu Bong-Chul, Loebel A
Rajagopalan K1, Wade SW2*, Chu Bong-Chul3 and Loebel A4
1Sunovion Pharmaceuticals Inc., Waterford Drive, Marlborough, MA, USA
2Wade Outcomes Research and Consulting, Salt Lake City, UT, USA
3Truven Health Analytics, Hollister Avenue, Santa Barbara, CA, USA
4Sunovion Pharmaceuticals Inc., One Bridge Plaza North, Fort Lee, NJ, USA
- Corresponding Author:
- Wade SW
Wade Outcomes Research and Consulting
358 South 700 East, Suite B-432
Salt Lake City, UT, USA
Tel: 801 884 6172
E-mail: sally.wade@truvenhealth.com
Received Date: March 31, 2016, Accepted Date: May 5, 2016, Published Date: May 12, 2016
Copyright: © 2016 Rajagopalan K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Context: Nonadherence to atypical antipsychotics is pervasive and contributes to suboptimal outcomes in patients with bipolar disorder.
Objective: Treatment adherence and discontinuation rates of lurasidone and other atypical antipsychotics of Medicaid and commercially-insured patients with bipolar disorder were evaluated.
Design: Patients newly initiating atypical antipsychotic therapy for bipolar disorder were identified in the Truven Health’s MarketScan® Commercial Claims and Encounters (Commercial) and the MarketScan® Medicaid Multi-State (Medicaid) databases.
Patients: Patients were grouped into eight treatment cohorts based on the first agent filled between October 1, 2009 and March 31, 2012.
Main Outcome Measures: Adherence was measured by medication possession ratio (MPR), discontinuation rate, and mean time to discontinuation during a 6-month follow-up. Medicaid and commercial data were analyzed separately.
Results: Among commercially-insured patients, the mean (SD) MPR was significantly higher in the lurasidone cohort compared to the olanzapine cohort [0.512 (0.335) vs. 0.445 (0.320); p < 0.05]. Lurasidone patients were less likely to discontinue than olanzapine patients (61.4% vs. 70.5%; p < 0.05). Among Medicaid patients, the mean MPR for lurasidone (0.535) was significantly higher than among those treated with other atypical antipsychotics (0.418 - 0.461) (all p < 0.05). The percentage of lurasidone users discontinuing index therapy was significantly lower than those in all other cohorts except quetiapine XR (all p < 0.05). In both commercial and Medicaid populations, time to index therapy discontinuation did not differ significantly between treatment cohorts.
Conclusion: Commercially-insured bipolar patients initiating lurasidone had better adherence and lower discontinuation rates versus olanzapine. Among the Medicaid bipolar population, patients initiating lurasidone exhibited better adherence and lower discontinuation rates compared to patients initiating other atypical antipsychotics.
Keywords
Adherence; Atypical antipsychotics; Bipolar disorder; Commercial; Medicaid
Introduction
Bipolar disorder is a chronic and disabling psychiatric illness that is associated with a wide range of comorbid psychiatric and medical conditions [1]. The National Comorbidity Study reported a lifetime prevalence of nearly 4% for bipolar disorder in the United States (US) [1]. Bipolar disorder is costly to treat, with one recent study reporting that bipolar disorder was the most expensive behavioral health diagnosis in a population of 1.7 million individuals with employer-sponsored health insurance [2].
Atypical antipsychotic medications are a relatively new, increasingly prominent component of the treatment armamentarium for bipolar disorder [3,4]. While randomized clinical trials have demonstrated the clinical effectiveness of atypical antipsychotics in patients with bipolar disorder [5-7] treatment adherence, an important determinant of their effectiveness, has not been well-studied. Treatment adherence in bipolar disorder is challenging given the chronic remissionrelapse pattern of the disorder, and the risk of nonadherence is highest during manic episodes [8]. Patients with bipolar disorder are estimated to have suboptimal adherence (21 – 50%) to their therapy, with the adherence rate for atypical antipsychotics at 37.7% [9,10].
Non-adherence is a critical issue in the treatment of bipolar disorder because it adversely affects patient prognosis and outcomes (e.g., symptom reduction, relapse rates, rehospitalization, suicide attempts, and quality of life) [9,11-13]. Among patients with bipolar disorder, good medication adherence has been associated with up to a 27% reduction in the odds of all-cause hospitalizations, a 24% reduction in the odds of mental health related hospitalizations, and up to a 29% reduction in the risk of mental health related emergency room (ER) visits [13-15]. Additionally, nonadherence contributes to increased total and outpatient mental health expenditures [16] as well as work loss-related indirect costs [17].
Lurasidone, a second-generation antipsychotic, received approval from the US Food and Drug Administration in June 2013 for the treatment of depressive episodes associated with bipolar disorder as monotherapy and as adjunctive therapy with lithium or valproate [18]. Because this approval was so recent, real-world data comparing adherence with lurasidone versus other atypical antipsychotics among individuals with bipolar disorder are scarce [19]. This study was conducted to begin filling that knowledge gap by providing real-world data on adherence and discontinuation rates of different atypical antipsychotics compared to lurasidone among patients with bipolar disorder.
Methods
Data Source
This was a retrospective analysis of data from the Truven Health MarketScan® Commercial Claims and Encounters (Commercial) and the Truven Health MarketScan Medicaid Multi-State Database (Medicaid) for bipolar disorder patients. The study evaluated enrollment data and administrative claims data for services incurred between October 1, 2009 and March 31, 2012. The medical claims files include inpatient and outpatient services, service dates, provider reimbursement amounts, patient copayment and deductible amounts. The Commercial database is constructed from data provided by large employer-sponsored health plans from across the US and contains the healthcare experience of more than 40 million privately insured individuals covered under a variety of fee-forservice, fully capitated, and partially capitated health plans. The Medicaid database contains the pooled healthcare experience of approximately 10 million Medicaid enrollees each year from multiple geographically dispersed states.
Patient selection
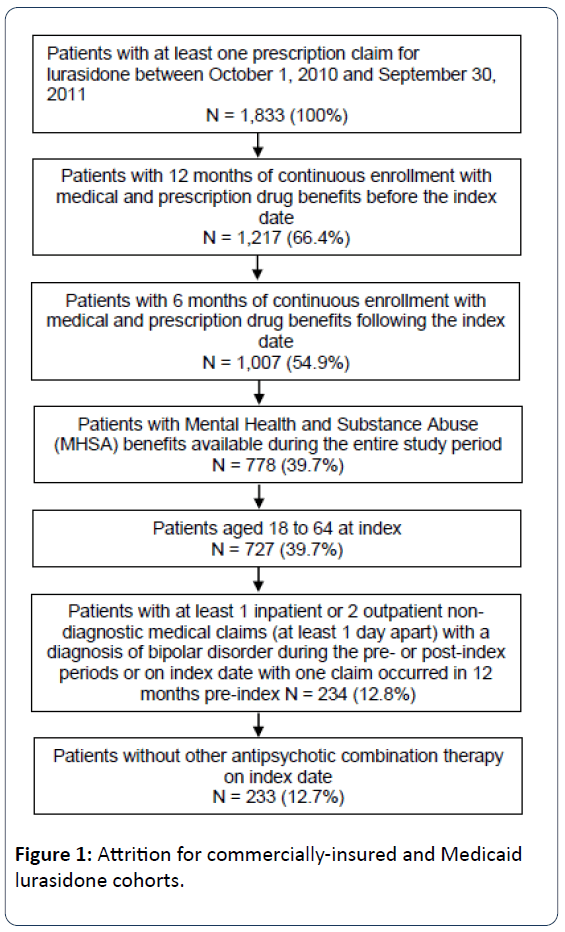
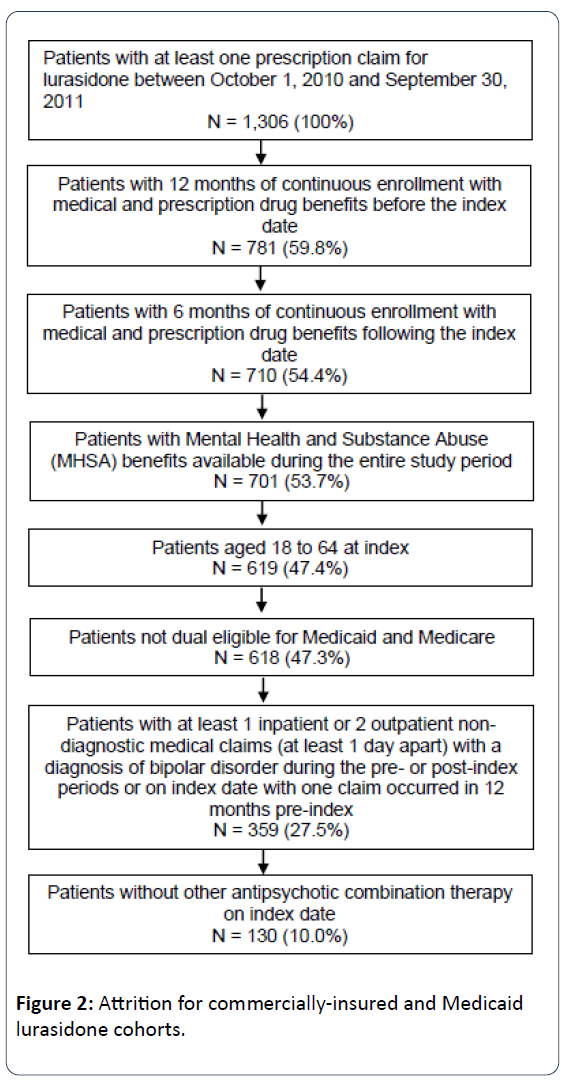
Adult patients (aged 18-64 years) newly initiating atypical antipsychotic therapy with lurasidone, aripiprazole, olanzapine, quetiapine (immediate release [IR], and extended release [XR]), risperidone or ziprasidone were selected for analysis. Specific oral antipsychotic cohorts were then created, with the lurasidone cohort selected first to maximize the cohort size. The date of the first prescription for antipsychotic therapy was the index date. Patients were required to have at least one inpatient or two outpatient claims at least one day apart with an International Classification of Diseases, Clinical Modification, Ninth Revision (ICD-9-CM) diagnosis code for bipolar disorder (296.0x, 296.1x, 296.4x, 296.8x, 301.11, 301.13) in any position on the claim during the study period; at least one claim in the 12 months prior to the index date. Continuous enrollment in a health insurance plan with mental health coverage was required of all patients for at least 12 months before and 6 months after the index date. Patients with dual eligibility for Medicare and Medicaid, and those patients whose mental health services were provided by third party vendors were excluded from the analysis, as their data may be incomplete within the Medicaid database. Additionally, patients were required to be on antipsychotic monotherapy at index and to be newly initiated (i.e., no use in the prior 6 months) on the index antipsychotic agent. These criteria were used to select all study patients, and their stepwise application is illustrated for the lurasidone population in Figures 1 and 2.
Patients were grouped into six cohorts, based on the atypical antipsychotic used at index (intent-to-treat): lurasidone, aripiprazole, olanzapine, quetiapine, risperidone or ziprasidone. The study period included 18 months of data for each patient: comprised of a 12-month history period to assess eligibility and classify patients, an index date (described previously), and 6-month post-index or follow-up period during which study outcomes were measured. The 6 months immediately prior to the index date was defined as the preindex period and the 6 months following the index date was designated as the post-index period.
Demographic characteristics and comorbidities
Demographic data, defined as of the index date, included age and gender. Patients covered by Medicaid were also described by race (white, black, non-white Hispanic, and other) and federal aid category used to qualify for eligibility (aged, blind, or disabled; child/family; and other). Commercially-insured patients were described by insurance plan type (comprehensive, exclusive provider organization or preferred provider organization, health management organization, point-of-service, consumer driven health plan or high deductible health plan, and unknown) and by U.S. Census Bureau region of residence (Northeast, North Central, South, West, and unknown).
The Charlson comorbidity index (CCI) (Deyo version), the number and percentage of patients with selected comorbidities (below), and the number and percentage of patients with specific psychiatric medication use were assessed for all patients in the six months immediately prior to the index date. Comorbidities of interest were identified using International Classification of Diseases, Ninth Edition-Clinical Modification diagnosis codes [ICD-9-CM] and included alcohol abuse, anxiety, depression, diabetes, dyslipidemia, hypertension, and obesity/central obesity. Psychiatric medication classes included antidepressants, antiparkinsons, anxiolytics, atypical antipsychotics, hypnotics/insomnia medications, migraine medications, mood stabilizers, muscle relaxants, stimulants, and typical antipsychotics. For patients covered by Medicaid, the percentage of patients with capitated claims in the 6 months subsequent to the index date was also reported.
Outcome measures
The primary study outcome was adherence to the index atypical antipsychotic therapy. Adherence was measured in three ways: medication possession ratio (MPR), discontinuation rate, and mean time to discontinuation. MPR was defined as the ratio of the total number of days of supply of the index therapy in the post-index period to the number of days in the post-index period. Discontinuation was determined by a gap of ≥45 days between index antipsychotic refills. Discontinuation rate was defined as the proportion of patients who discontinue their index antipsychotic therapy in the postindex period, and the mean time to discontinuation was also determined for these patients. Length of continuous therapy of index antipsychotic (in days) was also examined in the postindex period. Length of continuous therapy of the index medication was defined as days on therapy with no evidence of gaps ≥45 days between index antipsychotic refills.
Statistics
Descriptive analyses were conducted on all patient characteristics and adherence outcomes and were compared between lurasidone patients and those patients in the other antipsychotic treatment cohorts. Continuous measures were presented as means and standard deviations. Categorical measures were presented as percentages. Statistical tests of significance for differences in measures between the lurasidone cohort and other antipsychotic cohorts were performed. Chi-square tests were used to evaluate differences for categorical variables and Student’s t-tests were used to evaluate differences for continuous variables.
Since patient characteristics and medical and pharmacy benefits structures are likely to differ in fundamental ways between Medicaid-insured and commercially-insured populations, the adherence data were analyzed separately and population-specific results were reported in parallel. Although, it was beyond the scope of this study to directly compare adherence outcomes for patients in these two populations, both sets of results are presented to provide insights into real world adherence in these two different populations.
Results
Baseline demographics characteristics and comorbidities
Within the commercially-insured sample, a total of 233 lurasidone patients were included after all selection criteria were applied (Figure 1). Sample sizes for the comparison antipsychotics cohorts ranged from 635 patients (ziprasidone) to 2,519 patients (aripiprazole) (Table 1).
Table 1: Commercial: Demographic characteristics and comorbidities among bipolar patients.
| Lurasidone | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | |
|---|---|---|---|---|---|---|
| Patients - N | 233 | 2,519 | 801 | 2,365 | 1,147 | 635 |
| Female | 68.7% | 71.0% | 52.4%* | 63.9% | 58.9%* | 71.3% |
| Age, mean (SD) | 41.5 (12.3) | 40.3 (13.2) | 40.0 (14.1) | 40.3 (13.2) | 40.7 (13.8) | 41.0 (12.9) |
| Age Group | * | * | ||||
| 18-24 | 12.9% | 18.4% | 21.7% | 18.8% | 20.1% | 16.2% |
| 25-34 | 15.0% | 15.4% | 16.4% | 16.0% | 14.4% | 15.4% |
| 35-44 | 29.6% | 24.9% | 19.5% | 23.0% | 20.6% | 25.8% |
| 45-54 | 25.8% | 24.5% | 21.3% | 25.2% | 25.6% | 24.7% |
| 55-64 | 16.7% | 16.9% | 21.1% | 16.9% | 19.3% | 17.8% |
| Insurance Plan Type | * | * | * | * | * | |
| Comprehensive | 2.1% | 3.0% | 3.6% | 3.3% | 3.1% | 3.1% |
| EPO or PPO | 3.4% | 1.9% | 1.5% | 1.9% | 1.7% | 1.4% |
| HMO | 21.5% | 30.0% | 30.7% | 31.5% | 31.7% | 30.7% |
| Point-of-service | 58.8% | 55.5% | 53.6% | 53.4% | 52.8% | 54.2% |
| CDHP or HDHP | 8.2% | 5.9% | 6.2% | 5.2% | 5.8% | 6.3% |
| Unknown | 6.0% | 3.7% | 4.4% | 4.7% | 4.9% | 4.3% |
| US Census Geographic Region | * | * | ||||
| Northeast | 13.3% | 17.7% | 17.9% | 18.5% | 18.1% | 15.3% |
| North Central | 25.3% | 25.4% | 22.5% | 25.2% | 24.8% | 27.2% |
| South | 38.6% | 32.9% | 28.3% | 31.0% | 30.9% | 37.3% |
| West | 22.7% | 23.1% | 30.6% | 24.4% | 25.8% | 20.0% |
| Unknown | 0.0% | 0.8% | 0.7% | 1.0% | 0.4% | 0.2% |
| Charlson Comorbidity Index, mean (SD) | 0.4 (0.8) | 0.3 (0.7) | 0.3 (0.9) | 0.3 (0.8) | 0.3 (0.9) | 0.4 (0.9) |
| Prevalent Comorbidities | ||||||
| Alcohol Abuse | 5.2% | 6.9% | 9.5%* | 9.8%* | 10.4%* | 5.7% |
| Anxiety | 24.0% | 20.5% | 24.0% | 23.8% | 21.0% | 22.0% |
| Depression | 61.4% | 50.8%* | 47.6%* | 50.3%* | 48.9%* | 57.0% |
| Diabetes | 20.2% | 11.6%* | 8.4%* | 9.9%* | 12.2%* | 14.2%* |
| Dyslipidemia | 12.4% | 6.8%* | 7.2%* | 6.6%* | 7.6%* | 7.4%* |
| Hypertension | 20.2% | 13.4%* | 16.0% | 16.1% | 16.8% | 18.6% |
| Obesity / Central Obesity | 9.0% | 4.8%* | 3.0%* | 4.5%* | 4.8%* | 5.2%* |
| Pre-index Psychiatric Medication Use | ||||||
| Antidepressants | 69.5% | 66.0% | 55.2%* | 63.3% | 56.4%* | 64.4% |
| Antiparkinsons | 20.6% | 18.0% | 13.9%* | 13.9%* | 13.1%* | 18.0% |
| Anxiolytics | 65.2% | 49.3%* | 47.4%* | 53.8%* | 47.2%* | 55.6%* |
| Atypical antipsychotics | 73.4% | 7.5%* | 12.6%* | 6.2%* | 10.4%* | 15.1%* |
| Hypnotics/insomnia medications | 29.2% | 18.8%* | 18.6%* | 22.7%* | 18.0%* | 23.5% |
| Migraine medications | 8.6% | 4.8%* | 3.5%* | 4.8%* | 2.9%* | 3.8%* |
| Mood Stabilizers | 73.0% | 57.2%* | 50.6%* | 58.4%* | 58.1%* | 62.8%* |
| Muscle relaxants | 12.9% | 13.7% | 13.7% | 15.2% | 12.3% | 16.1% |
| Stimulants | 68.7% | 53.0%* | 43.2%* | 53.9%* | 51.4%* | 57.8%* |
| Typical antipsychotics | 6.9% | 0.4%* | 0.9%* | 0.7%* | 0.9%* | 0.9%* |
* P-value of lurasidone vs. antipsychotic in column significant at p < 0.05.
CDHP: Consumer-Directed Health Plan; EPO: Exclusive Provider Organization; HDHP: High Deductible Health Plan; HMO: Health Maintenance Organization; IR: Immediate-Release; PPO: Preferred Provider Organization; SD: Standard Deviation; XR: Extended-Release
The mean age across all commercial cohorts ranged between 39.6 and 41.5 years and was highest in the lurasidone cohort (41.5 years). The majority of patients in the lurasidone and other cohorts were female (52.4%−71.3%), resided in the Southern US Census Region (28.3%−38.6%) and were most commonly covered by a point-of-service (51.8%−58.8%) or health maintenance organization (21.5%−33.6%) plan. On average, patients treated with lurasidone had a slightly higher mean (SD) CCI score (0.4 [0.8]) compared with patients in the other cohorts, although the differences were not statistically significant at p < 0.05.
In all cohorts, most patients had evidence of other mental disorders, including depression (47.6%−61.4%) and anxiety (20.5%−25.9%) with significantly higher rates of depression observed in the lurasidone cohort (p < 0.05). Diabetes, dyslipidemia, and obesity were more common in the lurasidone cohort compared to all other antipsychotic cohorts (all p < 0.05). Psychiatric medications were commonly used in the pre-index period with highest rates of utilization observed in the lurasidone cohort.
The percentage of patients receiving both atypical and typical antipsychotics in the pre-index period was significantly higher in the lurasidone cohort compared to the other cohorts. In addition, lurasidone patients were more likely to use hypnotics, stimulants and mood stabilizers during the preperiod compared to those receiving other atypical antipsychotics (Table 1).
Within the Medicaid sample, a total of 130 lurasidone patients were included (Figure 2). Sample sizes for the comparison antipsychotics cohorts ranged from 289 patients (olanzapine) to 1,146 patients (quetiapine) (Table 2).
Table 2: Medicaid: Demographic characteristics and comorbidities among bipolar patients.
| Lurasidone | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | |
|---|---|---|---|---|---|---|
| Patients - N | 130 | 865 | 289 | 1,146 | 843 | 350 |
| Female | 75.4% | 77.9% | 66.4% | 74.3% | 73.8% | 82.9% |
| Age, mean (SD) | 34.2 (12.0) | 34.9 (11.7) | 36.4 (11.7) | 36.2 (11.4) | 34.7 (11.8) | 36.4 (11.5) |
| Age Group | * | * | * | |||
| 18-24 | 32.3% | 23.7% | 18.7% | 17.8% | 24.4% | 18.3% |
| 25-34 | 19.2% | 28.9% | 30.1% | 32.2% | 26.9% | 28.9% |
| 35-44 | 24.6% | 24.4% | 24.9% | 23.4% | 26.7% | 24.9% |
| 45-54 | 16.9% | 16.8% | 19.0% | 19.5% | 16.3% | 22.3% |
| 55-64 | 6.9% | 6.2% | 7.3% | 7.1% | 5.7% | 5.7% |
| Race | * | |||||
| White | 80.8% | 84.9% | 76.1% | 74.8% | 65.8% | 69.1% |
| Black | 12.3% | 9.9% | 15.6% | 17.7% | 25.6% | 20.3% |
| Non-white Hispanic | 0.8% | 0.9% | 0.3% | 0.7% | 0.8% | 1.1% |
| Other | 6.2% | 4.3% | 8.0% | 6.8% | 7.7% | 9.4% |
| Federal Aid Category | * | * | * | |||
| Aged, blind or disabled | 78.5% | 58.8% | 67.1% | 61.7% | 53.7% | 68.3% |
| Child/family | 20.0% | 38.7% | 30.8% | 36.5% | 44.5% | 30.0% |
| Other | 1.5% | 2.4% | 2.1% | 1.8% | 1.8% | 1.7% |
| Patient had any capitated claims during follow-up | 50.8% | 52.5% | 55.7% | 56.7% | 62.4%* | 60.6% |
| Charlson Comorbidity Index, mean (SD) | 0.6 (1.1) | 0.6 (1.2) | 0.7 (1.4) | 0.7 (1.3) | 0.6 (1.2) | 0.6 (1.0) |
| Prevalent Comorbidities | ||||||
| Alcohol Abuse | 6.9% | 9.6% | 14.2%* | 14.6%* | 10.6% | 12.9% |
| Anxiety | 24.6% | 33.2% | 38.1%* | 37.3%* | 31.2% | 29.7% |
| Depression | 57.7% | 56.5% | 51.9% | 54.5% | 50.5% | 58.3% |
| Diabetes | 29.2% | 20.9%* | 15.6%* | 19.3%* | 21.1%* | 28.0% |
| Dyslipidemia | 10.8% | 8.8% | 9.7% | 8.9% | 8.7% | 10.3% |
| Hypertension | 23.8% | 18.7% | 21.5% | 23.8% | 25.5% | 26.9% |
| Obesity / Central Obesity | 15.4% | 11.0% | 8.0%* | 10.2% | 10.3% | 15.4% |
| Pre-index Psychiatric Medication Use | ||||||
| Antidepressants | 67.7% | 64.5% | 56.7%* | 59.2% | 59.5% | 60.6% |
| Antiparkinsons | 12.3% | 8.0% | 8.0% | 6.0%* | 6.9%* | 5.7%* |
| Anxiolytics | 55.4% | 53.8% | 55.4% | 54.5% | 50.1% | 51.4% |
| Atypical antipsychotics | 78.5% | 8.1%* | 13.1%* | 8.5%* | 8.4%* | 12.6%* |
| Hypnotics/insomnia medications | 30.0% | 15.8%* | 17.3%* | 17.2%* | 15.4%* | 19.4%* |
| Migraine medications | 6.2% | 3.9% | 2.1%* | 4.5% | 4.0% | 6.6% |
| Mood Stabilizers | 66.2% | 47.9%* | 46.7%* | 43.6%* | 47.4%* | 57.4% |
| Muscle relaxants | 20.0% | 26.5% | 23.9% | 26.5% | 26.2% | 28.9% |
| Stimulants | 63.1% | 44.7%* | 43.3%* | 41.1%* | 45.6%* | 52.3%* |
| Typical antipsychotics | 13.8% | 1.0%* | 2.4%* | 1.0%* | 0.8%* | 0.9%* |
* P-value of lurasidone vs. antipsychotic in column significant at p < 0.05.
CDHP: Consumer-Directed Health Plan; EPO: Exclusive Provider Organization; HDHP: High Deductible Health Plan; HMO: Health Maintenance Organization; IR: Immediate-Release; PPO: Preferred Provider Organization; SD: Standard Deviation; XR: Extended-Release
In the Medicaid population, lurasidone patients had a mean age of 34.2 years, while in the other atypical antipsychotic cohorts the mean age ranged between 34.7 and 36.5 years.
In general, a greater percentage of lurasidone patients were in the 18−24 years age group (32.3% versus 16.6% to 24.4%), and differences were significant for the quetiapine and ziprasidone cohorts (p < 0.05). The mean CCI scores were similar across all cohorts, although the prevalence of specific comorbid conditions varied. Depression, diabetes, anxiety and hypertension were the most prominent comorbidities across all the cohorts. Patients in the lurasidone cohort had a significantly higher prevalence of diabetes (29.2%) compared to those in other cohorts, except ziprasidone. The rates of utilization of various psychiatric medications (i.e., atypical antipsychotics, hypnotics/insomnia medications, mood stabilizers, stimulants and typical antipsychotics) in the preindex period were often significantly higher among lurasidone patients compared with patients using other agents (Table 2).
Adherence
Commercially-insured lurasidone patients had significantly higher mean (SD) MPR compared to patients in the olanzapine cohort (0.512 [0.335] vs. 0.445 [0.320]; p < 0.05). In addition, a greater percentage of lurasidone patients achieved MPR of at least 0.8 compared with patients in the olanzapine cohort (30.5% vs. 22.7%, p < 0.05). However, the mean MPR and percentage of patients achieving MPR of at least 0.8 did not differ significantly between lurasidone and the other atypical antipsychotics cohorts. The percentage of patients in the lurasidone cohort who discontinued therapy was significantly lower than that among patients treated with olanzapine (61.4% vs. 70.5%; p < 0.05) while it did not differ significantly compared to patients in the other treatment cohorts. The average time to index therapy discontinuation was similar all across the cohorts (range: 50.3-57.0 days) (Table 3).
Table 3: Commercial: Descriptive adherence measures among bipolar patients.
| Lurasidone | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | |
|---|---|---|---|---|---|---|
| Patients – N | 233 | 2,519 | 801 | 2,365 | 1,147 | 635 |
| Medication Possession Ratio (MPR) during entire post-index period(Mean, SD) | 0.512 (0.335) | 0.520 (0.320) | 0.445 (0.320)* | 0.500 (0.338) | 0.498 (0.326) | 0.495 (0.339) |
| Patients with MPR ≥0.80 | 30.5% | 29.5% | 22.7%* | 29.6% | 28.2% | 29.4% |
| Patients who Discontinued3 Index Therapy | 61.4% | 61.1% | 70.5%* | 62.9% | 64.7% | 65.8% |
| Time to Discontinuation (days) (Mean, SD) | 53.0 (33.5) | 57.0 (33.9) | 50.3 (32.2) | 51.0 (32.4) | 54.7 (34.1) | 52.8 (35.0) |
* P-value of lurasidone vs. antipsychotic in column significant at p < 0.05.
In the Medicaid analysis, lurasidone patients had significantly higher mean (SD] MPR (0.535 [0.356]) than patients treated with other atypical antipsychotics (0.418 [0.324] to 0.461 [0.329], all p < 0.05). Patients in lurasidone cohort spent significantly more days (mean [SD]: 96.3 [64.0]) on their index therapy compared to patients using other atypical antipsychotics (mean [SD] 75.3 [58.3] to 83.1 [59.3] days, all p < 0.05) (not reported in table).
The percentage of lurasidone patients who discontinued index therapy (54.6%) was significantly lower compared to those in other treatment cohorts (range: 64.0−69.2%, all p < 0.05). There was no significant difference observed in time to discontinuation across the treatment cohorts (range: 44.5−49.8 days) (Table 4).
Table 4: Medicaid: Descriptive adherence measures among bipolar patients.
| Lurasidone | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | |
|---|---|---|---|---|---|---|
| Patients - N | 130 | 865 | 289 | 1,146 | 843 | 350 |
| Medication Possession Ratio (MPR) during entire post-index period(Mean, SD) | 0.535 (0.356) | 0.455 (0.325)* | 0.418 (0.324)* | 0.461 (0.329)* | 0.437 (0.310)* | 0.422 (0.324)* |
| Patients with MPR ≥0.80 | 36.9% | 23.4%* | 21.1%* | 24.9%* | 21.0%* | 23.1%* |
| Patients who Discontinued3 Index Therapy | 54.6% | 64.0%* | 69.2%* | 64.9%* | 68.8%* | 69.1%* |
| Time to Discontinuation (days) (Mean, SD) | 46.4 (32.0) | 47.9 (33.5) | 44.7 (32.0) | 49.1 (32.3) | 49.8 (32.4) | 44.5 (30.1) |
Discussion
This retrospective study examined the real-world adherence of lurasidone in comparison to other commonly prescribed atypical antipsychotic medications (i.e., aripiprazole, olanzapine, quetiapine, risperidone and ziprasidone) in Medicaid and commercially-insured patients with bipolar disorder. In the commercially-insured population, adherence with lurasidone was better (i.e., higher MPR and lower discontinuation rate) in comparison to olanzapine and comparable to that observed for the other atypical antipsychotic agents studied. In the Medicaid population, lurasidone patients exhibited a better adherence profile (i.e., higher mean MPR, a greater percentage of patients achieving MPR of at least 0.8, and lower discontinuation rate) compared to bipolar patients treated with other atypical antipsychotics.
Few prior studies have compared adherence with atypical antipsychotic therapies among patients with bipolar disorder, and the follow-up periods assessed varied from 6 to 12 months [10,13,18-20]. The mean MPRs (0.51 – 0.54 for lurasidone, and 0.42 – 0.52 for the other atypical antipsychotics) observed in our two study populations fall within the mid-range of MPR reported in studies conducted prior to the introduction of lurasidone. The wide range of mean MPR values reported in those studies (0.19 – 0.71) reflects varied sample sizes and study designs, diverse patient populations, and differences in follow-up periods assessed [10,13,18-20]. Even when study designs and populations appear similar, reported adherence may vary. For example, during a 12-month follow-up period, one Medicaid study reported a relatively high mean MPR range of 0.68 – 0.71 for bipolar patients using olanzapine, risperidone, and quetiapine [18], while another Medicaid study reported a much lower mean MPR of 0.19 for bipolar patients using aripiprazole, olanzapine, quetiapine, and risperidone [16]. This variation is not unexpected since the underlying populations and benefits structures of Medicaid claims databases used for research vary depending on which states are represented, and other details of study design of differences in patient characteristics, may also have contributed to these inconsistent findings.
In the literature, four commercial insurance-based studies have reported a lower range of mean MPR (0.21 – 0.46) for patients using atypical antipsychotic [10,13,18,21] than the current study. In our study, commercially-insured patients on lurasidone showed a similar discontinuation rate of 61.4% compared to most other atypical antipsychotic patients (60.3 – 65.8%), but a lower rate compared with olanzapine patients (70.5%). In the current study, among Medicaid patients the discontinuation rate was 54.6% for lurasidone patients, with higher discontinuation rates (64.0 – 69.2%) observed among patients using other atypical antipsychotics. Chen et al. reported a similar discontinuation rates for commerciallyinsured (64.4%) and Medicaid (62.8%) patients using atypical antipsychotics for bipolar disorder [16]. The mean time to discontinuation that we observed for lurasidone patients falls within the range observed for patients who were using other atypical antipsychotic in both the commercially-insured and Medicaid populations that we studied (53 days vs. 50.3 – 57.0 days in commercially-insured; 46.4 days vs. 44.7 – 49.8 days in Medicaid). By contrast, Chen et al. reported a slightly longer time to discontinuation at 66 days using a 12-month follow-up period [16].
Nonadherence is a critical issue in the management of bipolar disorder because it is an important predictor of hospitalization risk [10]. Medication adherence has been shown to significantly reduce the risk of rehospitalization among patients with mental illness, particularly bipolar disorder [13-15]. The results of a study by Hassan and colleagues [13] indicate that patients with bipolar disorder (93% commercial, ~3% Medicaid, ~4% other insurance) who achieved an MPR threshold of 0.75 had a 27% reduction in the odds of all-cause hospitalization and a 24% reduction in the odds of mental-health related hospitalization. In another study, as patients with bipolar disorder (93% commercial, ~3% Medicaid, ~4% other insurance) achieved a higher MPR threshold, the odds of emergency room visits, hospitalization for any cause and mental health-related hospitalizations, decreased ; Lage et al.[14] reported that an MPR ≥ 0.75 was associated with a reduced risk of an ER visit for any cause (odds ratio [OR] 0.84, 95% confidence interval [CI] 0.74 to 0.96) or hospitalization for any cause (OR 0.85, 95% CI 0.75 to 0.98) and an MPR ≥ 0.80 was associated with a significant reduction in the risk of a mental health-related hospitalization (OR 0.82, 95% CI 0.70 to 0.95), while an MPR ≥ 0.90 was associated with a significant reduction in risk of a mental health-related ER visits (OR 0.71, 95% CI 0.54 to 0.91) [20]. Similarly, in a cohort of Medicaid patients followed over a 12- month period, discontinuation of antipsychotic medication for as little as 1–10 days was found to nearly double the risk of hospitalization, with a gap of over 30 days resulting in an approximately fourfold increased risk of hospitalization [21].
Reducing hospitalization risk is not only important from a clinical and patient perspective, but also from an economic perspective since bipolar disorder associated hospitalizations are costly. In a 2002 analysis of a national inpatient sample, the U.S. Department of Health and Human Services found the mean length of stay for depression or bipolar disorder related hospitalization to be 7.9 days, resulting in an average cost of $11,500 [22]. In another study, the mean cost of a single psychiatric hospitalization was estimated at $9,635 (2004 USD) for patients with bipolar disorder, while the mean cost of a hospitalization stay for any reason for patients with bipolar disorder was estimated at $11,500 (2002 USD) in one study and $16,609 (2004 USD) in another study [13].
This study did not compare adherence in the Medicaid and commercial populations, because patient characteristics and insurance benefits structures that influence adherence levels are likely different between these two populations. For example, insurance type has been shown to impact adherence [23], and demographic and clinical characteristic differences of the patients eligible for each type of benefit may potentially shape patterns of medication use. Multiple sociodemographic factors, including gender [24], race/ethnicity [25], and face-toface pharmacy counseling [26], may also directly impact adherence. Lower educational levels, African American race, and substance abuse have been associated with nonadherence [27-31] among bipolar patients. Patients insured through Medicaid have a greater prevalence of mental disorders and other chronic diseases [32] compared to commercial patients, which may also increase the risk of nonadherence. A guidelinerelated review of the mental health literature concluded that a ‘positive relationship with clinical staff’ was a significant predictor of good adherence among patients with serious mental health conditions [31]. Similarly, ‘difficulties in building a therapeutic alliance’ and ‘poor clinician–patient relationship’ were predictors of nonadherence. Compared to commerciallyinsured patients, Medicaid patients are likely to receive lower quality of care and to rely more on acute care services [33], therefore it might be expected that Medicaid patients have less positive relationships with clinicians. Medicaid patients are also more likely to have risk factors for nonadherence such as chaotic living conditions, and financial or logistical problems (e.g., transportation) than commercially-insured patients. The two populations may also have differences in social and family support, which are associated with better adherence [31].
It is noteworthy that, in the Medicaid population, adherence with lurasidone was significantly better than adherence with the other atypical antipsychotics. The differences in adherence patterns between Medicaid and commercial bipolar populations may be of interest for future research, but could prove to be challenging as many of the factors shown to be influence adherence to antipsychotics, such as beliefs about medication benefits and side effects, are difficult to measure and adjust for in comparative analysis.
Several limitations to the analyses in our study merit consideration. First, the MarketScan® research databases rely on the accuracy of data entered for purposes related to the business of healthcare, rather than according to strict research protocols. Second, the Medicaid analyses may not generalize well to patients with dual Medicare/Medicaid eligibility (excluded from the study population) because those patients receive their drug coverage through Medicaid rather than Medicare. Third, the analyses were limited to those patients who were continuously enrolled thereby limiting generalizability of the findings to the wider population. This may be especially true for the Medicaid population since individuals enter and exit Medicaid plans frequently. Fourth, since psychiatric inpatient treatment provided in psychiatric hospitals with 16 or more beds is not reimbursed by Medicaid, the analysis might primarily reflect psychiatric inpatient care provided in general hospital psychiatric units. On a related note, Medicaid patients receiving psychiatric treatment but paid for through mechanisms other than Medicaid were not included in the analysis therefore introducing selection bias.
In summary, results of the current study suggest that Medicaid patients receiving lurasidone treatment for bipolar disorder had better adherence and overall lower discontinuation rates compared to patients receiving other atypical antipsychotic drugs. Among commercially-insured patients, lurasidone adherence and discontinuation rates were better when compared with olanzapine, but similar to other atypical antipsychotics. Future studies using similar measures of adherence in larger cohort of patients may help further confirm these study findings. Future studies using similar measures of adherence in larger cohort of patients are warranted to confirm or refute the findings of the present study.
Acknowledgement
The authors thank Santosh Tiwari and Nicole Meyer (Truven Health) and Pankaj Patel (Sunovion Pharmaceuticals) for their contributions to manuscript preparation, and Larry Radbill (Truven Health at the time of the study) for his assistance with analyses and programming. Final decisions regarding manuscript content were made jointly by the authors. S. Wade, K. Rajagopalan and A. Loebel were the primary developers of the study design, and N. Meyer conducted data analyses. K. Rajagopalan had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors assisted in interpreting the study findings and drafting the manuscript text, all authors provided critical input to the content of the manuscript, and all were responsible for approving the manuscript and its contents.
Conflict of interest statement
K. Rajagopalan and A. Loebel are employees of Sunovion Pharmaceuticals, Inc. S. Wade is an employee of Wade Outcomes Research and Consulting and worked as an independent contractor for Truven Health Analytics to conduct this study. B. Chu is an employee of Truven Health Analytics. Truven Health received funding from Sunovion Pharmaceuticals to conduct this study. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
References
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617-627.
- Peele PB, Xu Y, Kupfer DJ (2003) Insurance expenditures on bipolar disorder: clinical and parity implications. Am J Psychiatry 160: 1286-1290.
- American Psychiatric Association (2002) Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 15: 1-50.
- Keck PE, Otto MW, Carpenter D, Ross R, Docherty JP (2004) The expert consensus guideline series: treatment of bipolar disorder 2004. Postgraduate Medicine Special Report,pp: 1-116.
- Sachs G, Chengappa KN, Suppes T, Mullen JA, Brecher M, et al. (2004) Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord 6: 213-223.
- Tohen M, Chengappa KN, Suppes T, Zarate CA, Calabrese JR, et al. (2002) Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 59: 62-69.
- Yatham LN, Binder C, Riccardelli R, Leblanc J, Connolly M, et al. (2003) Risperidone in acute and continuation treatment of mania. IntClinPsychopharmacol 18: 227-235.
- Velligan D, Sajatovic M, Valenstein M, Riley WT, Safren S, et al. (2010) Methodological challenges in psychiatric treatment adherence research. ClinSchizophrRelat Psychoses 4: 74-91.
- Buckley PF, Patel NC, Wermert A (2009) Adherence to mental health treatment. New York: Oxford University Press 1: 53-69.
- Berger A, Sanders KN, Alvir JM, Mychaskiw MA, Oster G (2012) Medication adherence and utilization in patients with schizophrenia or bipolar disorder receiving aripiprazole, quetiapine, or ziprasidone at hospital discharge: a retrospective cohort study. BMC Psychiatry 12: 99.
- Colom F, Vieta E, Tacchi MJ, Sanchez-Moreno J, Scott J (2005) Identifying and improving non-adherence in bipolar disorders. Bipolar Disord 5: 24-31.
- El-Mallakh RS (2007) Medication adherence and the use of long-acting antipsychotics in bipolar disorder. J PsychiatrPract 13: 79-85.
- Hassan M, Lage MJ (2009) Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge. Am J Health Syst Pharm 66: 358-365.
- Lage MJ, Hassan MK (2009) The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: a retrospective study. Ann Gen Psychiatry 8: 7.
- Weiden PJ, Kozma C, Grogg A, Locklear J (2004) Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. PsychiatrServ 55: 886-891.
- Gianfrancesco FD, Sajatovic M, Rajagopalan K, Wang RH (2008) Antipsychotic treatment adherence and associated mental health care use among individuals with bipolar disorder. ClinTher 30: 1358-1374.
- Bagalman E, Yu-Isenberg KS, Durden E, Crivera C, Dirani R, et al. (2010) Indirect costs associated with nonadherence to treatment for bipolar disorder. J Occup Environ Med 52: 478-485.
- Latuda® (lurasidone) Prescribing Information (2014) Sunovion Pharmaceuticals Inc. Marlborough, MA.
- Perlick DA, Rosenheck RA, Kaczynski R, Kozma L (2014) Medication non-adherence in bipolar disorder: A patient-centered review of research findings 2014; Worthing, ROYAUME-UNI: Cambridge Medical Publications.
- Chen W, Deveaugh-Geiss AM, Palmer L, Princic N, Chen YT (2013) Patterns of atypical antipsychotic therapy use in adults with bipolar I disorder in the USA. Hum Psychopharmacol 28: 428-437.
- Hassan M, Madhavan SS, Kalsekar ID, Makela EH, Rajagopalan K, et al (2007) Comparing adherence to and persistence with antipsychotic therapy among patients with bipolar disorder. Ann Pharmacother 4: 1812-1818.
- Agency for Healthcare Research and Quality (2002) Statistics on stays in U.S. hospitals, principal diagnosis. Number of discharges, mean length of stay, mean charges, percent admitted from the ED, and percent died in the hospital. HCUP Fact Book No 6: hospitalization in the United States.
- Yeaw J, Benner JS, Walt JG, Sian S, Smith DB (2009) Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 15: 728-740.
- Gibson TB, Mark TL, McGuigan KA, Axelsen K, Wang S (2006) The effects of prescription drug copayments on statin adherence. Am J Manag Care 12: 509-517.
- Gerber BS, Cho YI, Arozullah AM, Lee SY (2010) Racial differences in medication adherence: A cross-sectional study of Medicare enrollees. Am J GeriatrPharmacother 8: 136-145.
- Ishisaka DY, Jukes T, Romanelli RJ, Wong KS, Schiro TA (2012) Disparities in adherence to and persistence with antihypertensive regimens: an exploratory analysis from a community-based provider network. J Am SocHypertens 6: 201-209.
- Janssen B, Gaebel W, Haerter M, Komaharadi F, Lindel B, et al. (2006) Evaluation of factors influencing medication compliance in inpatient treatment of psychotic disorders. Psychopharmacology (Berl) 187: 229-236.
- Baldessarini RJ, Perry R, Pike J (2008) Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol 23: 95-105.
- Copeland LA, Zeber JE, Salloum IM, Pincus HA, Fine MJ, et al.(2008) Treatment adherence and illness insight in veterans with bipolar disorder. J NervMent Dis 196: 16-21.
- Johnson FR, Ozdemir S, Manjunath R, Hauber AB, Burch SP, et al. (2007) Factors that affect adherence to bipolar disorder treatments: a stated-preference approach. Med Care 45: 545-552.
- Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, et al. (2009) The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry 70: 1-46.
- Gibson TB, Lee TA, Vogeli CS, Hidalgo J, Carls GS, et al. (2009) A four-system comparison of patients with chronic illness: the Military Health System, Veterans Health Administration, Medicaid, and commercial plans. Mil Med 174: 936-943.
- Landon BE, Schneider EC, Normand SL, Scholle SH, Pawlson LG, et al. (2007) Quality of care in Medicaid managed care and commercial health plans. JAMA 298: 1674-1681.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences